Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(beta-L-malic acid) for drug delivery
- PMID: 16536461
- PMCID: PMC3487710
- DOI: 10.1021/bc0502457
Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(beta-L-malic acid) for drug delivery
Abstract
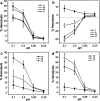
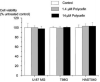
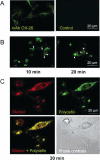
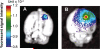
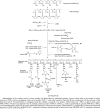
A new prototype of nanoconjugate, Polycefin, was synthesized for targeted delivery of antisense oligonucleotides and monoclonal antibodies to brain tumors. The macromolecular carrier contains: 1. biodegradable, nonimmunogenic, nontoxic beta-poly(L-malic acid) of microbial origin; 2. Morpholino antisense oligonucleotides targeting laminin alpha4 and beta1 chains of laminin-8, which is specifically overexpressed in glial brain tumors; 3. monoclonal anti-transferrin receptor antibody for specific tissue targeting; 4. oligonucleotide releasing disulfide units; 5. L-valine containing, pH-sensitive membrane disrupting unit(s), 6. protective poly(ethylene glycol); 7. a fluorescent dye (optional). Highly purified modules were conjugated directly with N-hydroxysuccinimidyl ester-activated beta-poly(L-malic acid) at pendant carboxyl groups or at thiol containing spacers via thioether and disulfide bonds. Products were chemically validated by physical, chemical, and functional tests. In vitro experiments using two human glioma cell lines U87MG and T98G demonstrated that Polycefin was delivered into the tumor cells by a receptor-mediated endocytosis mechanism and was able to inhibit the synthesis of laminin-8 alpha4 and beta1 chains at the same time. Inhibition of laminin-8 expression was in agreement with the designed endosomal membrane disruption and drug releasing activity. In vivo imaging showed the accumulation of intravenously injected Polycefin in brain tumor tissue via the antibody-targeted transferrin receptor-mediated endosomal pathway in addition to a less efficient mechanism known for high molecular mass biopolymers as enhanced permeability and retention effect. Polycefin was nontoxic to normal and tumor astrocytes in a wide range of concentrations, accumulated in brain tumor, and could be used for specific targeting of several biomarkers simultaneously.
Figures


Similar articles
-
Brain tumor tandem targeting using a combination of monoclonal antibodies attached to biopoly(beta-L-malic acid).J Control Release. 2007 Oct 8;122(3):356-63. doi: 10.1016/j.jconrel.2007.05.032. Epub 2007 Jun 5. J Control Release. 2007. PMID: 17630012 Free PMC article.
-
Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis.Angiogenesis. 2006;9(4):183-91. doi: 10.1007/s10456-006-9046-9. Epub 2006 Nov 16. Angiogenesis. 2006. PMID: 17109197 Free PMC article.
-
Nanoconjugate based on polymalic acid for tumor targeting.Chem Biol Interact. 2008 Jan 30;171(2):195-203. doi: 10.1016/j.cbi.2007.01.015. Epub 2007 Feb 8. Chem Biol Interact. 2008. PMID: 17376417 Free PMC article.
-
Poly(malic acid) nanoconjugates containing various antibodies and oligonucleotides for multitargeting drug delivery.Nanomedicine (Lond). 2008 Apr;3(2):247-65. doi: 10.2217/17435889.3.2.247. Nanomedicine (Lond). 2008. PMID: 18373429 Free PMC article. Review.
-
Natural and synthetic poly(malic acid)-based derivates: a family of versatile biopolymers for the design of drug nanocarriers.J Drug Target. 2014 Aug;22(7):556-75. doi: 10.3109/1061186X.2014.936871. J Drug Target. 2014. PMID: 25012064 Review.
Cited by
-
Preparation of Two Types of Polymeric Micelles Based on Poly(β-L-Malic Acid) for Antitumor Drug Delivery.PLoS One. 2016 Sep 20;11(9):e0162607. doi: 10.1371/journal.pone.0162607. eCollection 2016. PLoS One. 2016. PMID: 27649562 Free PMC article.
-
Inhibition of brain tumor growth by intravenous poly (β-L-malic acid) nanobioconjugate with pH-dependent drug release [corrected].Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18143-8. doi: 10.1073/pnas.1003919107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921419 Free PMC article.
-
Covalent nano delivery systems for selective imaging and treatment of brain tumors.Adv Drug Deliv Rev. 2017 Apr;113:177-200. doi: 10.1016/j.addr.2017.06.002. Epub 2017 Jun 10. Adv Drug Deliv Rev. 2017. PMID: 28606739 Free PMC article. Review.
-
Multifunctional Self-Assembled Films for Rapid Hemostat and Sustained Anti-infective Delivery.ACS Biomater Sci Eng. 2015 Mar 9;1(3):148-156. doi: 10.1021/ab500050m. Epub 2015 Feb 25. ACS Biomater Sci Eng. 2015. PMID: 33429517 Free PMC article.
-
Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-L-malic acid).Pharm Res. 2010 Nov;27(11):2317-29. doi: 10.1007/s11095-010-0091-0. Epub 2010 Apr 13. Pharm Res. 2010. PMID: 20387095 Free PMC article.
References
-
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 2001;53:283–318. - PubMed
-
- Christie RJ, Grainger DW. Design strategies to improve soluble macromolecular delivery constructs. Adv. Drug Delivery Rev. 2003;55:421–437. - PubMed
-
- Duncan R. The dawning era of polymer therapeutics. Nat Rev. 2003;2:347–360. - PubMed
-
- Zhang Y, Zhu C, Pardridge WM. Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol. Ther. 2002;6:67–72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

