A novel ternary ligand system useful for preparation of cationic (99m)Tc-diazenido complexes and (99m)Tc-labeling of small biomolecules
- PMID: 16536480
- PMCID: PMC2597566
- DOI: 10.1021/bc0502715
A novel ternary ligand system useful for preparation of cationic (99m)Tc-diazenido complexes and (99m)Tc-labeling of small biomolecules
Abstract
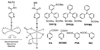
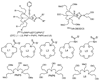
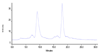
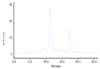
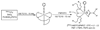
This report describes a novel ternary ligand system composed of a phenylhydrazine, a crown ether-containing dithiocarbamate (DTC), and a PNP-type bisphosphine (PNP). The combination of three different ligands with (99m)Tc results in cationic (99m)Tc-diazenido complexes, [(99m)Tc(NNAr)(DTC)(PNP)]+, with potential radiopharmaceuticals for heart imaging. Synthesis of cationic (99m)Tc-diazenido complexes can be accomplished in two steps. For example, the reaction of phenylhydrazine with (99m)TcO4- at 100 degrees C in the presence of excess stannous chloride and 1,2-diaminopropane-N,N,N',N'-tetraacetic acid (PDTA) results in the [(99m)Tc(NNPh)(PDTA)n] intermediate, which then reacts with sodium N-(dithiocarbamato)-2-aminomethyl-15-Crown-5 (L4) and N,N-bis[2-(bis(3-ethoxypropyl)phosphino)ethyl]ethoxyethylamine (PNP6) at 100 degrees C for 15 min to give the complex, [(99m)Tc(NNPh)(L4)(PNP6)]+ in high yield (>90%). Cationic complexes [(99m)Tc(NNPh)(DTC)(PNP)]+ are stable for > or = 6 h. Their composition was determined to be 1:1:1:1 for Tc:NNPh:DTC:PNP using the mixed-ligand experiments on the tracer ((99m)Tc) level and was further confirmed by the ESI-MS spectral data of a model compound [Re(NNPh)(L4)(L6)]+. It was found that both DTCs and bisphosphines have a significant impact on the lipophilicity of their cationic (99m)Tc-diazenido complexes. Results from a (99m)Tc-labeling efficiency experiment showed that 4-hydrazinobenzoic acid (HYBA) might be useful as a bifunctional coupling agent for (99m)Tc-labeling of small biomolecules. However, the (99m)Tc-labeling efficiency of HYBA is much lower than that of 6-hydrazinonicotinic acid (HYNIC) with tricine and trisodium triphenylphosphine-3,3',3''-trisulfonate (TPPTS) as coligands.
Figures








References
-
- Abrams MJ, Juweid M, tenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss HW, Fischman AJ. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J. Nucl. Med. 1990;31:2022–2028. - PubMed
-
- Schwartz DA, Abrams MJ, Hauser MM, Gaul FE, Larsen SK, Rauh D, Zubieta J. Preparation of hydrazino-modified proteins and their use for the synthesis of 99mTc-protein conjugates. Bioconjugate Chem. 1991;2:333–336. - PubMed
-
- Ultee ME, Bridger GJ, Abrams MJ, Longley CB, Burton CA, Larsen SK, Henson GW, Padmanabhan S, Gaul FE, Schwartz DA. Tumor imaging with technetium-99m-labeled hydrazinonicotinamide-Fab' conjugates. J. Nucl. Med. 1997;38:133–138. - PubMed
-
- Bridger GJ, Abrams MJ, Padmanabhan S, Gaul FE, Larsen S, Henson GW, Schwartz DA, Longley CB, Burton CA, Ultee ME. A comparison of cleavable and noncleavable hydrazinopyridine linkers for the 99mTc-labeling of Fab’ monoclonal antibody fragments. Bioconjugate Chem. 1996;7:255–264. - PubMed
-
- Babich JW, Solomon H, Pike MC, Kroon D, Graham W, Abrams MJ, Tompkins RG, Rubin RH, Fischman AJ. Technetium-99m labeled hydrazino nicotinamide derivatized chemotactic peptide analogs for imaging focal sites of bacterial infection. J. Nucl. Med. 1993;34:1964–1974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

