High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis
- PMID: 16537378
- PMCID: PMC1450222
- DOI: 10.1073/pnas.0511304103
High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis
Abstract
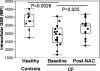
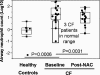
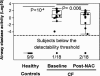
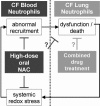
Neutrophilic airway inflammation is a hallmark of cystic fibrosis (CF). As high oxidant producers, airway neutrophils contribute largely to the systemic redox imbalance seen in CF. In turn, this chronic and profound imbalance can impact circulating neutrophils before their migration into airways. Indeed, in 18 CF patients with stable disease, blood neutrophils were readily deficient in the pivotal antioxidant glutathione (P = 0.003, compared with 9 healthy controls). In a phase 1 study, this deficiency was improved (P = 0.025) by the glutathione prodrug N-acetylcysteine, given orally in high doses (0.6 to 1.0 g three times daily, for 4 weeks). This treatment was safe and markedly decreased sputum elastase activity (P = 0.006), the strongest predictor of CF pulmonary function. Consistently, neutrophil burden in CF airways was decreased upon treatment (P = 0.003), as was the number of airway neutrophils actively releasing elastase-rich granules (P = 0.005), as measured by flow cytometry. Pulmonary function measures were not improved, as expected with short-term treatment. After excluding data from subjects without baseline airway inflammation, positive treatment effects were more pronounced and included decreased sputum IL-8 levels (P = 0.032). Thus, high-dose oral N-acetylcysteine has the potential to counter the intertwined redox and inflammatory imbalances in CF.
Conflict of interest statement
Conflict of interest statement: R.T., C.K.C., Leonore A. Herzenberg, R.B.M., and Leonard A. Herzenberg are listed as inventors on a provisional patent application covering NAC as a therapeutic agent for CF. Leonore A. Herzenberg and Leonard A. Herzenberg hold a small amount of equity in BioAdvantex (Mississauga, ON, Canada), which sells European GMP NAC and provided the NAC used in this study.
Figures

References
-
- Davis P. B. Pediatr. Rev. 2001;22:257–264. - PubMed
-
- Cantin A. Am. J. Respir. Crit. Care Med. 1995;151:939–941. - PubMed
-
- Khan T. Z., Wagener J. S., Bost T., Martinez J., Accurso F. J., Riches D. W. Am. J. Respir. Crit. Care Med. 1995;151:1075–1082. - PubMed
-
- Tirouvanziam R., Khazaal I., Peault B. Am. J. Physiol. 2002;283:L445–L451. - PubMed
-
- Muhlebach M. S., Stewart P. W., Leigh M. W., Noah T. L. Am. J. Respir. Crit. Care Med. 1999;160:186–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

