Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2
- PMID: 16537386
- PMCID: PMC1450242
- DOI: 10.1073/pnas.0509378103
Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2
Abstract
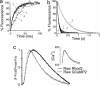
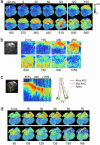
Genetically encoded sensor proteins provide unique opportunities to advance the understanding of complex cellular interactions in physiologically relevant contexts; however, previously described sensors have proved to be of limited use to report cell signaling in vivo in mammals. Here, we describe an improved Ca(2+) sensor, GCaMP2, its inducible expression in the mouse heart, and its use to examine signaling in heart cells in vivo. The high brightness and stability of GCaMP2 enable the measurement of myocyte Ca(2+) transients in all regions of the beating mouse heart and prolonged pacing and mapping studies in isolated, perfused hearts. Transgene expression is efficiently temporally regulated in cardiomyocyte GCaMP2 mice, allowing recording of in vivo signals 4 weeks after transgene induction. High-resolution imaging of Ca(2+) waves in GCaMP2-expressing embryos revealed key aspects of electrical conduction in the preseptated heart. At embryonic day (e.d.) 10.5, atrial and ventricular conduction occur rapidly, consistent with the early formation of specialized conduction pathways. However, conduction is markedly slowed through the atrioventricular canal in the e.d. 10.5 heart, forming the basis for an effective atrioventricular delay before development of the AV node, as rapid ventricular activation occurs after activation of the distal AV canal tissue. Consistent with the elimination of the inner AV canal muscle layer at e.d. 13.5, atrioventricular conduction through the canal was abolished at this stage. These studies demonstrate that GCaMP2 will have broad utility in the dissection of numerous complex cellular interactions in mammals, in vivo.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Berridge M. J., Irvine R. F. Nature. 1984;312:315–321. - PubMed
-
- Tsien R. W., Tsien R. Y. Annu. Rev. Cell Biol. 1990;6:715–760. - PubMed
-
- Bootman M. D., Berridge M. J. Cell. 1995;83:675–678. - PubMed
-
- Clapham D. E. Cell. 1995;80:259–268. - PubMed
-
- Berridge M. J., Bootman M. D., Lipp P. Nature. 1998;395:645–648. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

