Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies
- PMID: 16537393
- PMCID: PMC1450215
- DOI: 10.1073/pnas.0505379103
Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies
Abstract
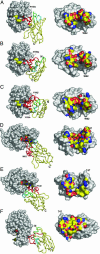
Clefts on protein surfaces are avoided by antigen-combining sites of conventional antibodies, in contrast to heavy-chain antibodies (HCAbs) of camelids that seem to be attracted by enzymes' substrate pockets. The explanation for this pronounced preference of HCAbs was investigated. Eight single domain antigen-binding fragments of HCAbs (VHH) with nanomolar affinities for lysozyme were isolated from three immunized dromedaries. Six of eight VHHs compete with small lysozyme inhibitors. This ratio of active site binders is also found within the VHH pool derived from polyclonal HCAbs purified from the serum of the immunized dromedary. The crystal structures of six VHHs in complex with lysozyme and their interaction surfaces were compared to those of conventional antibodies with the same antigen. The interface sizes of VHH and conventional antibodies to lysozyme are very similar as well as the number and chemical nature of the contacts. The main difference comes from the compact prolate shape of VHH that presents a large convex paratope, predominantly formed by the H3 loop and interacting, although with different structures, into the concave lysozyme substrate-binding pocket. Therefore, a single domain antigen-combining site has a clear structural advantage over a conventional dimeric format for targeting clefts on antigenic surfaces.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Padlan E. A. Adv. Protein Chem. 1996;49:57–133. - PubMed
-
- MacCallum R. M., Martin A. C., Thornton J. M. J. Mol. Biol. 1996;262:732–745. - PubMed
-
- Al Lazikani B., Lesk A. M., Chothia C. J. Mol. Biol. 1997;273:927–948. - PubMed
-
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R., et al. Nature. 1989;342:877–883. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

