SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons
- PMID: 16537406
- PMCID: PMC1450173
- DOI: 10.1073/pnas.0600084103
SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons
Abstract
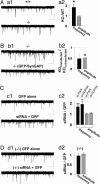
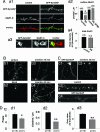
Silent synapses, or excitatory synapses that lack functional alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), are thought to be critical for regulation of neuronal circuits and synaptic plasticity. Here, we report that SynGAP, an excitatory synapse-specific RasGAP, regulates AMPAR trafficking, silent synapse number, and excitatory synaptic transmission in hippocampal and cortical cultured neurons. Overexpression of SynGAP in neurons results in a remarkable depression of AMPAR-mediated miniature excitatory postsynaptic currents, a significant reduction in synaptic AMPAR surface expression, and a decrease in the insertion of AMPARs into the plasma membrane. This change is specific for AMPARs because no change is observed in synaptic NMDA receptor expression or total synapse density. In contrast to these results, synaptic transmission is increased in neurons from SynGAP knockout mice as well as in neuronal cultures treated with SynGAP small interfering RNA. In addition, activation of the extracellular signal-regulated kinase, ERK, is significantly decreased in SynGAP-overexpressing neurons, whereas P38 mitogen-activated protein kinase (MAPK) signaling is potentiated. Furthermore, ERK activation is up-regulated in neurons from SynGAP knockout mice, whereas P38 MAPK function is depressed. Taken together, these data suggest that SynGAP plays a critical role in the regulation of neuronal MAPK signaling, AMPAR membrane trafficking, and excitatory synaptic transmission.
Conflict of interest statement
Conflict of interest statement: Under a licensing agreement between Upstate Group, Inc. and the Johns Hopkins University, R.L.H. is entitled to a share of royalty received by the University on sales of products described in this article. R.L.H. is a paid consultant to Upstate Group, Inc. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Figures




Comment in
-
Profile of Richard L. Huganir.Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4341-3. doi: 10.1073/pnas.0601079103. Epub 2006 Mar 13. Proc Natl Acad Sci U S A. 2006. PMID: 16537364 Free PMC article. No abstract available.
Similar articles
-
SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation.Neuron. 2004 Aug 19;43(4):563-74. doi: 10.1016/j.neuron.2004.08.003. Neuron. 2004. PMID: 15312654
-
SynGAP regulates protein synthesis and homeostatic synaptic plasticity in developing cortical networks.PLoS One. 2013 Dec 31;8(12):e83941. doi: 10.1371/journal.pone.0083941. eCollection 2013. PLoS One. 2013. PMID: 24391850 Free PMC article.
-
SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor.J Neurosci. 2002 Nov 15;22(22):9721-32. doi: 10.1523/JNEUROSCI.22-22-09721.2002. J Neurosci. 2002. PMID: 12427827 Free PMC article.
-
Twenty Years of SynGAP Research: From Synapses to Cognition.J Neurosci. 2020 Feb 19;40(8):1596-1605. doi: 10.1523/JNEUROSCI.0420-19.2020. J Neurosci. 2020. PMID: 32075947 Free PMC article. Review.
-
AMPAR trafficking in synapse maturation and plasticity.Cell Mol Life Sci. 2013 Dec;70(23):4411-30. doi: 10.1007/s00018-013-1309-1. Epub 2013 Mar 9. Cell Mol Life Sci. 2013. PMID: 23475111 Free PMC article. Review.
Cited by
-
Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome.Nat Neurosci. 2014 Dec;17(12):1736-43. doi: 10.1038/nn.3863. Epub 2014 Nov 10. Nat Neurosci. 2014. PMID: 25383899 Free PMC article.
-
Autism spectrum disorder: neuropathology and animal models.Acta Neuropathol. 2017 Oct;134(4):537-566. doi: 10.1007/s00401-017-1736-4. Epub 2017 Jun 5. Acta Neuropathol. 2017. PMID: 28584888 Free PMC article. Review.
-
A Subset of Autism-Associated Genes Regulate the Structural Stability of Neurons.Front Cell Neurosci. 2016 Nov 17;10:263. doi: 10.3389/fncel.2016.00263. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27909399 Free PMC article. Review.
-
Synaptic plasticity through activation of GluA3-containing AMPA-receptors.Elife. 2017 Aug 1;6:e25462. doi: 10.7554/eLife.25462. Elife. 2017. PMID: 28762944 Free PMC article.
-
SynGAP is expressed in the murine suprachiasmatic nucleus and regulates circadian-gated locomotor activity and light-entrainment capacity.Eur J Neurosci. 2021 Feb;53(3):732-749. doi: 10.1111/ejn.15043. Epub 2020 Nov 30. Eur J Neurosci. 2021. PMID: 33174316 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

