Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism
- PMID: 16537448
- PMCID: PMC1450135
- DOI: 10.1073/pnas.0600092103
Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism
Abstract
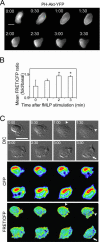
Chemoattractants like fMet-Leu-Phe (fMLP) induce neutrophils to polarize with phosphatidylinositol 3,4,5-trisphosphate (PIP3) and protrusive F-actin at the front and actomyosin contraction at the sides and back. RhoA and its downstream effector, myosin II, mediate the "backness" response, which locally inhibits the "frontness" response and constrains its location to one part of the cell. In living HL-60 cells, we used a fluorescent PIP3 probe or a single-chain FRET biosensor for RhoA-GTP to assess spatial distribution of frontness or backness responses, respectively, during the first 3 min after exposure to a uniform concentration of fMLP. Increased PIP3 signal or RhoA activity initially localized randomly about the cell's periphery but progressively redistributed to the front or to the back and sides, respectively. Cells rendered unable to mount the frontness response (by inhibiting actin polymerization or Gi, a trimeric G protein) responded to a micropipette source of attractant by localizing RhoA activity at the up-gradient edge. We infer that protrusive F-actin, induced by the frontness response, constrains the spatial distribution of backness by locally reducing activation of RhoA, thereby reducing its active form at the front. Mutual incompatibility of frontness and backness is responsible for self-organization of neutrophil polarity.
Conflict of interest statement
Conflict of interest statement: K.H. is a paid consultant of Genospectra (Fremont, CA).
Figures


References
-
- Hauert A. B., Martinelli S., Marone C., Niggli V. Int. J. Biochem. Cell Biol. 2002;34:838–854. - PubMed
-
- Merlot S., Firtel R. A. J. Cell Sci. 2003;116:3471–3478. - PubMed
-
- Chung C. Y., Funamoto S., Firtel R. A. Trends Biochem. Sci. 2001;26:557–566. - PubMed
-
- Raftopoulou M., Hall A. Dev. Biol. 2004;265:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

