Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures
- PMID: 16537456
- PMCID: PMC1533783
- DOI: 10.1073/pnas.0511323103
Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures
Abstract
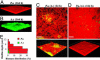
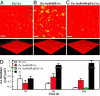
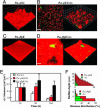
In the environment, multiple microbial taxa typically coexist as communities, competing for resources and, often, physically associated within biofilms. A dual-species cocultivation model has been developed by using two ubiquitous and well studied microbes Pseudomonas aeruginosa (P.a.) and Agrobacterium tumefaciens (A.t.) as a tractable system to identify molecular mechanisms that underlie multispecies microbial associations. Several factors were found to influence coculture interactions. P.a. had a distinct growth-rate advantage in cocultures, increasing its relative abundance during planktonic and biofilm growth. P.a. also demonstrated a slight quorum-sensing-dependent increase in growth yield in liquid cocultures. P.a. dominated coculture biofilms, "blanketing" or burying immature A.t. microcolonies. P.a. flagellar and type IV pili mutant strains exhibited deficient blanketing and impaired competition in coculture biofilms, whereas, in planktonic coculture, these mutations had no effect on competition. In contrast, A.t. used motility to emigrate from coculture biofilms. In both planktonic and biofilm cocultures, A.t. remained viable for extended periods of time, coexisting with its more numerous competitor. These findings reveal that quorum-sensing-regulated functions and surface motility are important microbial competition factors for P.a. and that the outcome of competition and the relative contribution of different factors to competition are strongly influenced by the environment in which they occur.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

