Cyclosporin and Timothy syndrome increase mode 2 gating of CaV1.2 calcium channels through aberrant phosphorylation of S6 helices
- PMID: 16537462
- PMCID: PMC1533789
- DOI: 10.1073/pnas.0511322103
Cyclosporin and Timothy syndrome increase mode 2 gating of CaV1.2 calcium channels through aberrant phosphorylation of S6 helices
Abstract
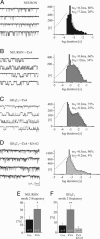
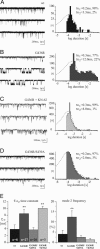
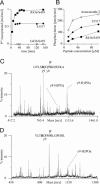
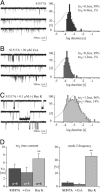
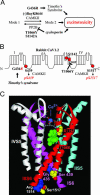
Calcium channels in the plasma membrane rarely remain open for much more than a millisecond at any one time, which avoids raising intracellular calcium to toxic levels. However, the dihydropyridine-sensitive calcium channels of the CaV1 family, which selectively couple electrical excitation to endocrine secretion, cardiovascular contractility, and neuronal transcription, have a unique second mode of gating, "mode 2," that involves frequent openings of much longer duration. Here we report that two human conditions, cyclosporin neurotoxicity and Timothy syndrome, increase mode 2 gating of the recombinant rabbit CaV1.2 channel. In each case, mode 2 gating depends on a Ser residue at the cytoplasmic end of the S6 helix in domain I (Ser-439, Timothy syndrome) or domain IV (Ser-1517, cyclosporin). Both Ser reside in consensus sequences for type II calmodulin-dependent protein kinase. Pharmacologically inhibiting type II calmodulin-dependent protein kinase or mutating the Ser residues to Ala prevents the increase in mode 2 gating. We propose that aberrant phosphorylation, or "phosphorylopathy," of the CaV1.2 channel protein contributes to the excitotoxicity associated with Timothy syndrome and with chronic cyclosporin treatment of transplant patients.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Similar articles
-
Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome.Circ Res. 2010 Mar 5;106(4):748-56. doi: 10.1161/CIRCRESAHA.109.213363. Epub 2010 Jan 28. Circ Res. 2010. PMID: 20110531 Free PMC article.
-
Basal and β-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700.Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16598-603. doi: 10.1073/pnas.1419129111. Epub 2014 Nov 3. Proc Natl Acad Sci U S A. 2014. PMID: 25368181 Free PMC article.
-
Effect of the Brugada syndrome mutation A39V on calmodulin regulation of Cav1.2 channels.Mol Brain. 2014 Apr 28;7:34. doi: 10.1186/1756-6606-7-34. Mol Brain. 2014. PMID: 24775099 Free PMC article.
-
Hallmarks of the channelopathies associated with L-type calcium channels: a focus on the Timothy mutations in Ca(v)1.2 channels.Biochimie. 2011 Dec;93(12):2080-6. doi: 10.1016/j.biochi.2011.05.015. Epub 2011 May 31. Biochimie. 2011. PMID: 21664226 Review.
-
L-type CaV1.2 calcium channels: from in vitro findings to in vivo function.Physiol Rev. 2014 Jan;94(1):303-26. doi: 10.1152/physrev.00016.2013. Physiol Rev. 2014. PMID: 24382889 Review.
Cited by
-
PhosSNP for systematic analysis of genetic polymorphisms that influence protein phosphorylation.Mol Cell Proteomics. 2010 Apr;9(4):623-34. doi: 10.1074/mcp.M900273-MCP200. Epub 2009 Dec 8. Mol Cell Proteomics. 2010. PMID: 19995808 Free PMC article.
-
Molecular endpoints of Ca2+/calmodulin- and voltage-dependent inactivation of Ca(v)1.3 channels.J Gen Physiol. 2010 Mar;135(3):197-215. doi: 10.1085/jgp.200910308. Epub 2010 Feb 8. J Gen Physiol. 2010. PMID: 20142517 Free PMC article.
-
Pharmacological correction of gating defects in the voltage-gated Ca(v)2.1 Ca²⁺ channel due to a familial hemiplegic migraine mutation.Neuron. 2014 Jan 8;81(1):91-102. doi: 10.1016/j.neuron.2013.10.056. Neuron. 2014. PMID: 24411734 Free PMC article.
-
Ca(2+) current facilitation is CaMKII-dependent and has arrhythmogenic consequences.Front Pharmacol. 2014 Jun 17;5:144. doi: 10.3389/fphar.2014.00144. eCollection 2014. Front Pharmacol. 2014. PMID: 24987371 Free PMC article. Review.
-
Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival.J Biol Chem. 2010 Dec 24;285(52):41122-34. doi: 10.1074/jbc.M110.130351. Epub 2010 Sep 14. J Biol Chem. 2010. PMID: 20841359 Free PMC article.
References
-
- Chin D., Means A. R. Trends Cell Biol. 2000;10:322–328. - PubMed
-
- Catterall W. A. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. - PubMed
-
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. J. Mol. Cell Cardiol. 1986;18:691–710. - PubMed
-
- Dzhura I., Wu Y., Colbran R. J., Balser J. R., Anderson M. E. Nat. Cell Biol. 2000;2:173–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

