Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases
- PMID: 16537516
- PMCID: PMC1449678
- DOI: 10.1073/pnas.0511256103
Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases
Abstract
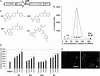
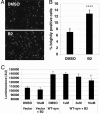
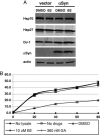
Misfolded proteins accumulate in many neurodegenerative diseases, including huntingtin in Huntington's disease and alpha-synuclein in Parkinson's disease. The disease-causing proteins can take various conformations and are prone to aggregate and form larger cytoplasmic or nuclear inclusions. One approach to the development of therapeutic intervention for these diseases has been to identify chemical compounds that reduce the size or number of inclusions. We have, however, identified a compound that promotes inclusion formation in cellular models of both Huntington's disease and Parkinson's disease. Of particular interest, this compound prevents huntingtin-mediated proteasome dysfunction and reduces alpha-synuclein-mediated toxicity. These results demonstrate that compounds that increase inclusion formation may actually lessen cellular pathology in both Huntington's and Parkinson's diseases, suggesting a therapeutic approach for neurodegenerative diseases caused by protein misfolding.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Frydman J. Annu. Rev. Biochem. 2001;70:603–647. - PubMed
-
- Glickman M. H., Ciechanover A. Physiol. Rev. 2002;82:373–428. - PubMed
-
- Ross C. A., Pickart C. M. Trends Cell Biol. 2004;14:703–711. - PubMed
-
- Arrasate M., Mitra S., Schweitzer E. S., Segal M. R., Finkbeiner S. Nature. 2004;431:805–810. - PubMed
-
- Bucciantini M., Giannoni E., Chiti F., Baroni F., Formigli L., Zurdo J., Taddei N., Ramponi G., Dobson C. M., Stefani M. Nature. 2002;416:507–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

