A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacterium tuberculosis
- PMID: 16537518
- PMCID: PMC1449680
- DOI: 10.1073/pnas.0510861103
A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacterium tuberculosis
Abstract
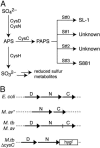
Sulfated molecules have been shown to modulate isotypic interactions between cells of metazoans and heterotypic interactions between bacterial pathogens or symbionts and their eukaryotic host cells. Mycobacterium tuberculosis, the causative agent of tuberculosis, produces sulfated molecules that have eluded functional characterization for decades. We demonstrate here that a previously uncharacterized sulfated molecule, termed S881, is localized to the outer envelope of M. tuberculosis and negatively regulates the virulence of the organism in two mouse infection models. Furthermore, we show that the biosynthesis of S881 relies on the universal sulfate donor 3'-phosphoadenosine-5'-phosphosulfate and a previously uncharacterized sulfotransferase, stf3. These findings extend the known functions of sulfated molecules as general modulators of cell-cell interactions to include those between a bacterium and a human host.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Farzan M., Mirzabekov T., Kolchinsky P., Wyatt R., Cayabyab M., Gerard N. P., Gerard C., Sodroski J., Choe H. Cell. 1999;96:667–676. - PubMed
-
- Moore K. L. J. Biol. Chem. 2003;278:24243–24246. - PubMed
-
- Kehoe J. W., Bertozzi C. R. Chem. Biol. 2000;7:R57–R61. - PubMed
-
- Choe H., Li W., Wright P. L., Vasilieva N., Venturi M., Huang C. C., Grundner C., Dorfman T., Zwick M. B., Wang L., Rosenberg E. S., et al. Cell. 2003;114:161–170. - PubMed
-
- Hirata T., Furukawa Y., Yang B. G., Hieshima K., Fukuda M., Kannagi R., Yoshie O., Miyasaka M. J. Biol. Chem. 2004;279:51775–51782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

