Orphaned ryanodine receptors in the failing heart
- PMID: 16537526
- PMCID: PMC1449688
- DOI: 10.1073/pnas.0509324103
Orphaned ryanodine receptors in the failing heart
Abstract
Heart muscle is characterized by a regular array of proteins and structures that form a repeating functional unit identified as the sarcomere. This regular structure enables tight coupling between electrical activity and Ca(2+) signaling. In heart failure, multiple cellular defects develop, including reduced contractility, altered Ca(2+) signaling, and arrhythmias; however, the underlying causes of these defects are not well understood. Here, in ventricular myocytes from spontaneously hypertensive rats that develop heart failure, we identify fundamental changes in Ca(2+) signaling that are related to restructuring of the spatial organization of the cells. Myocytes display both a reduced ability to trigger sarcoplasmic reticulum Ca(2+) release and increased spatial dispersion of the transverse tubules (TTs). Remodeled TTs in cells from failing hearts no longer exist in the regularly organized structures found in normal heart cells, instead moving within the sarcomere away from the Z-line structures and leaving behind the sarcoplasmic reticulum Ca(2+) release channels, the ryanodine receptors (RyRs). These orphaned RyRs appear to be responsible for the dyssynchronous Ca(2+) sparks that have been linked to blunted contractility and, probably, Ca(2+)-dependent arrhythmias in diverse models of heart failure. We conclude that the increased spatial dispersion of the TTs and orphaned RyRs lead to the loss of local control and Ca(2+) instability in heart failure.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
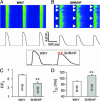

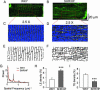
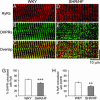
References
-
- Cheng H., Lederer W. J., Cannell M. B. Science. 1993;262:740–744. - PubMed
-
- Lopez-Lopez J. R., Shacklock P. S., Balke C. W., Wier W. G. Science. 1995;268:1042–1045. - PubMed
-
- Cannell M. B., Cheng H., Lederer W. J. Science. 1995;268:1045–1050. - PubMed
-
- Cheng H., Cannell M. B., Lederer W. J. Pflügers Arch. 1994;428:415–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

