Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1
- PMID: 16537601
- PMCID: PMC1440418
- DOI: 10.1128/JVI.80.7.3341-3348.2006
Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1
Abstract
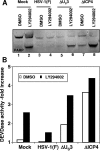
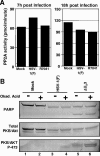
The product of the herpes simplex virus 1 (HSV-1) US3 gene is a multifunctional serine-threonine protein kinase that can block apoptosis induced by proapoptotic cellular proteins, exogenous agents, or replication-defective viruses. Earlier studies showed that the U(S)3 kinase activates and functionally overlaps cellular protein kinase A (PKA). In this study we examined the status of phosphatidylinositol 3-kinase [PI3K] and of its effector, protein kinase B/Akt (PKB/Akt), a component of a major pathway of mammalian antiapoptotic signaling systems. We report the following. (i) Infection of target cells with HSV-1 induces transient phosphorylation of serine 473 of PKB/Akt early in infection, with a mechanism that is dependent on PI3K. Inhibition of PI3K induced apoptosis in mock-infected or deltaU(S)3 mutant-virus-infected but not in wild-type-virus-infected cells and reduced the accumulation of specific viral gene products, including the U(S)3 protein kinase, but had a marginal effect on virus yields. (ii) At later times after infection, the total amounts of PKB/Akt decreased and phosphorylated PKB/Akt forms disappeared in a U(S)3-dependent and protein phosphatase 2A-independent manner. (iii) Activation of PKA by forskolin did not mediate significant dephosphorylation of PKB/Akt. Our results are consistent with the model that PKB/Akt is activated early in infection and acts to block apoptosis in infected cells prior to the accumulation of U(S)3 protein kinase and that it persists and continues to function as an antiapoptotic protein in the absence of U(S)3 but becomes redundant or even inimical once U(S)3 protein kinase accumulates in effective amounts.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

