Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13(II) is required for viral infectivity in vivo
- PMID: 16537614
- PMCID: PMC1440407
- DOI: 10.1128/JVI.80.7.3469-3476.2006
Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13(II) is required for viral infectivity in vivo
Abstract
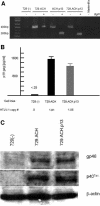
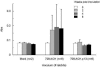
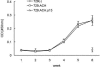
Human T-lymphotropic virus type 1 (HTLV-1), the etiological agent of adult T-cell leukemia, encodes unique regulatory and accessory proteins in the pX region of the provirus, including the open reading frame II product p13(II). p13(II) localizes to mitochondria, binds farnesyl pyrophosphate synthetase, an enzyme involved in posttranslational farnesylation of Ras, and alters Ras-dependent cell signaling and control of apoptosis. The role of p13(II) in virus infection in vivo remains undetermined. Herein, we analyzed the functional significance of p13(II) in HTLV-1 infection. We compared the infectivity of a human B-cell line that harbors an infectious molecular clone of HTLV-1 with a selective mutation that prevents the translation of p13(II) (729.ACH.p13) to the infectivity of a wild-type HTLV-1-expressing cell line (729.ACH). 729.ACH and 729.ACH.p13 producer lines had comparable infectivities for cultured rabbit peripheral blood mononuclear cells (PBMC), and the fidelity of the start codon mutation in ACH.p13 was maintained after PBMC passage. In contrast, zero of six rabbits inoculated with 729.ACH.p13 cells failed to establish viral infection, whereas six of six rabbits inoculated with wild-type HTLV-1-expressing cells (729.ACH) were infected as measured by antibody responses, proviral load, and HTLV-1 p19 matrix antigen production from ex vivo-cultured PBMC. Our data are the first to indicate that the HTLV-1 mitochondrion-localizing protein p13(II) has an essential biological role during the early phase of virus infection in vivo.
Figures


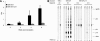

Similar articles
-
Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo.J Virol. 2000 Feb;74(3):1094-100. doi: 10.1128/jvi.74.3.1094-1100.2000. J Virol. 2000. PMID: 10627519 Free PMC article.
-
Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: evidence of in vivo reversion.J Virol. 2004 Apr;78(8):3837-45. doi: 10.1128/jvi.78.8.3837-3845.2004. J Virol. 2004. PMID: 15047799 Free PMC article.
-
In vitro and in vivo functional analysis of human T cell lymphotropic virus type 1 pX open reading frames I and II.AIDS Res Hum Retroviruses. 2000 Nov 1;16(16):1757-64. doi: 10.1089/08892220050193272. AIDS Res Hum Retroviruses. 2000. PMID: 11080823
-
Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis.Microbiol Mol Biol Rev. 2002 Sep;66(3):396-406, table of contents. doi: 10.1128/MMBR.66.3.396-406.2002. Microbiol Mol Biol Rev. 2002. PMID: 12208996 Free PMC article. Review.
-
Role of accessory proteins of HTLV-1 in viral replication, T cell activation, and cellular gene expression.Front Biosci. 2004 Sep 1;9:2556-76. doi: 10.2741/1417. Front Biosci. 2004. PMID: 15358581 Free PMC article. Review.
Cited by
-
Human T-lymphotropic virus type 1 non-structural proteins: Requirements for latent infection.Cancer Sci. 2013 Aug;104(8):983-8. doi: 10.1111/cas.12190. Epub 2013 Jun 17. Cancer Sci. 2013. PMID: 23651172 Free PMC article. Review.
-
De novo human T-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common gamma-chain knockout mice.J Virol. 2006 Nov;80(21):10683-91. doi: 10.1128/JVI.01009-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943297 Free PMC article.
-
Upregulation of mitochondrial gene expression in PBMC from convalescent SARS patients.J Clin Immunol. 2006 Nov;26(6):546-54. doi: 10.1007/s10875-006-9046-y. Epub 2006 Oct 6. J Clin Immunol. 2006. PMID: 17024565 Free PMC article.
-
Human T lymphotropic virus type 1 regulatory and accessory gene transcript expression and export are not rex dependent.AIDS Res Hum Retroviruses. 2012 Apr;28(4):405-10. doi: 10.1089/aid.2011.0130. Epub 2011 Aug 5. AIDS Res Hum Retroviruses. 2012. PMID: 21819218 Free PMC article.
-
The HTLV-1 hbz antisense gene indirectly promotes tax expression via down-regulation of p30(II) mRNA.Virology. 2011 Feb 20;410(2):307-15. doi: 10.1016/j.virol.2010.11.019. Epub 2010 Dec 21. Virology. 2011. PMID: 21176937 Free PMC article.
References
-
- Awasthi, S., A. Sharma, K. Wong, J. Zhang, E. F. Matlock, L. Rogers, P. Motloch, S. Takemoto, H. Taguchi, M. D. Cole, B. Lüscher, O. Dittrich, H. Tagami, Y. Nakatani, M. McGee, A.-M. Girard, L. Gaughan, C. N. Robson, R. J. Monnat, Jr., and R. Harrod. 2005. A human T-cell lymphotropic virus type 1 enhancer of Myc transforming potential stabilizes Myc-TIP60 transcriptional interactions. Mol. Cell. Biol. 25:6178-6198. - PMC - PubMed
-
- Basbous, J., C. Arpin, G. Gaudray, M. Piechaczyk, C. Devaux, and J. M. Mesnard. 2003. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J. Biol. Chem. 278:43620-43627. - PubMed
-
- Boya, P., A. L. Pauleau, D. Poncet, R. A. Gonzalez-Polo, N. Zamzami, and G. Kroemer. 2004. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta 1659:178-189. - PubMed
-
- Cereseto, A., Z. Berneman, I. Koralnik, J. Vaughn, G. Franchini, and M. E. Klotman. 1997. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia 11:866-870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

