UL26-deficient human cytomegalovirus produces virions with hypophosphorylated pp28 tegument protein that is unstable within newly infected cells
- PMID: 16537622
- PMCID: PMC1440364
- DOI: 10.1128/JVI.80.7.3541-3548.2006
UL26-deficient human cytomegalovirus produces virions with hypophosphorylated pp28 tegument protein that is unstable within newly infected cells
Abstract
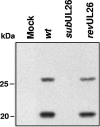
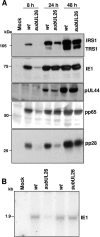
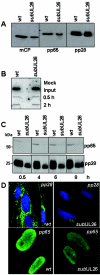
The human cytomegalovirus UL26 open reading frame encodes proteins of 21 and 27 kDa that result from the use of two different in-frame initiation codons. The UL26 protein is a constituent of the virion and thus is delivered to cells upon viral entry. We have characterized a mutant of human cytomegalovirus in which the UL26 open reading frame has been deleted. The UL26 deletion mutant has a profound growth defect, the magnitude of which is dependent on the multiplicity of infection. Two very early defects were discovered. First, even though they were present in normal amounts within mutant virions, the UL99-coded pp28 and UL83-coded pp65 tegument proteins were present in reduced amounts at the earliest times assayed within newly infected cells; second, there was a delay in immediate-early mRNA and protein accumulation. Further analysis revealed that although wild-type levels of the pp28 tegument protein were present in UL26 deletion mutant virions, the protein was hypophosphorylated. We conclude that the UL26 protein influences the normal phosphorylation of at least pp28 in virions and possibly additional tegument proteins. We propose that the hypophosphorylation of tegument proteins causes their destabilization within newly infected cells, perhaps disrupting the normal detegumentation process and leading to a delay in the onset of immediate-early gene expression.
Figures



Similar articles
-
Deletion of open reading frame UL26 from the human cytomegalovirus genome results in reduced viral growth, which involves impaired stability of viral particles.J Virol. 2006 Jun;80(11):5423-34. doi: 10.1128/JVI.02585-05. J Virol. 2006. PMID: 16699023 Free PMC article.
-
Open reading frame UL26 of human cytomegalovirus encodes a novel tegument protein that contains a strong transcriptional activation domain.J Virol. 2002 May;76(10):4836-47. doi: 10.1128/jvi.76.10.4836-4847.2002. J Virol. 2002. PMID: 11967300 Free PMC article.
-
Cytoplasmic envelopment of human cytomegalovirus requires the postlocalization function of tegument protein pp28 within the assembly compartment.J Virol. 2007 Jun;81(12):6536-47. doi: 10.1128/JVI.02852-06. Epub 2007 Mar 28. J Virol. 2007. PMID: 17392372 Free PMC article.
-
Tegument proteins of human cytomegalovirus.Microbiol Mol Biol Rev. 2008 Jun;72(2):249-65, table of contents. doi: 10.1128/MMBR.00040-07. Microbiol Mol Biol Rev. 2008. PMID: 18535146 Free PMC article. Review.
-
Human cytomegalovirus tegument proteins (pp65, pp71, pp150, pp28).Virol J. 2012 Jan 17;9:22. doi: 10.1186/1743-422X-9-22. Virol J. 2012. PMID: 22251420 Free PMC article. Review.
Cited by
-
Insights into the Transcriptome of Human Cytomegalovirus: A Comprehensive Review.Viruses. 2023 Aug 8;15(8):1703. doi: 10.3390/v15081703. Viruses. 2023. PMID: 37632045 Free PMC article. Review.
-
Cooperative inhibition of RIP1-mediated NF-κB signaling by cytomegalovirus-encoded deubiquitinase and inactive homolog of cellular ribonucleotide reductase large subunit.PLoS Pathog. 2017 Jun 1;13(6):e1006423. doi: 10.1371/journal.ppat.1006423. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28570668 Free PMC article.
-
UL49 is an essential subunit of the viral pre-initiation complex that regulates human cytomegalovirus gene transcription.iScience. 2022 Sep 19;25(10):105168. doi: 10.1016/j.isci.2022.105168. eCollection 2022 Oct 21. iScience. 2022. PMID: 36204275 Free PMC article.
-
Identification of compounds with anti-human cytomegalovirus activity that inhibit production of IE2 proteins.Antiviral Res. 2017 Feb;138:61-67. doi: 10.1016/j.antiviral.2016.12.006. Epub 2016 Dec 9. Antiviral Res. 2017. PMID: 27956134 Free PMC article.
-
The IκB Kinases Restrict Human Cytomegalovirus Infection.J Virol. 2019 Apr 17;93(9):e02030-18. doi: 10.1128/JVI.02030-18. Print 2019 May 1. J Virol. 2019. PMID: 30760575 Free PMC article.
References
-
- Anderson, M. L. M. 1999. Nucleic acid hybridization. Springer, New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

