Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species
- PMID: 16537623
- PMCID: PMC1440393
- DOI: 10.1128/JVI.80.7.3549-3558.2006
Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species
Abstract
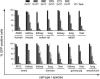
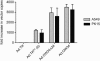
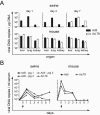
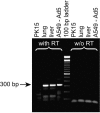
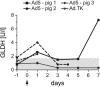
Oncolytic adenoviruses have emerged as a promising approach for the treatment of tumors resistant to other treatment modalities. However, preclinical safety studies are hampered by the lack of a permissive nonhuman host. Screening of a panel of primary cell cultures from seven different animal species revealed that porcine cells support productive replication of human adenovirus type 5 (Ad5) nearly as efficiently as human A549 cells, while release of infectious virus by cells from other animal species tested was diminished by several orders of magnitude. Restriction of productive Ad5 replication in rodent and rabbit cells seems to act primarily at a postentry step. Replication efficiency of adenoviral vectors harboring different E1 deletions or mutations in porcine cells was similar to that in A549 cells. Side-by-side comparison of the viral load kinetics in blood of swine and mice injected with Ad5 or a replication-deficient adenoviral vector failed to provide clear evidence for virus replication in mice. In contrast, evidence suggests that adenovirus replication occurs in swine, since adenoviral late gene expression produced a 13.5-fold increase in viral load in an individual swine from day 3 to day 7 and 100-fold increase in viral DNA levels in the Ad5-infected swine compared to the animal receiving a replication-deficient adenovirus. Lung histology of Ad5-infected swine revealed a severe interstitial pneumonia. Although the results in swine are based on a small number of animals and need to be confirmed, our data strongly suggest that infection of swine with human adenovirus or oncolytic adenoviral vectors is a more appropriate animal model to study adenoviral pathogenicity or pharmacodynamic and toxicity profiles of adenoviral vectors than infection of mice.
Figures



References
-
- Alemany, R., K. Suzuki, and D. T. Curiel. 2000. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 81:2605-2609. - PubMed
-
- Arola, M., O. Ruuskanen, T. Ziegler, and T. T. Salmi. 1995. Respiratory virus infections during anticancer treatment in children. Pediatr. Infect. Dis. J. 14:690-694. - PubMed
-
- Batshaw, M. L., J. M. Wilson, S. Raper, M. Yudkoff, and M. B. Robinson. 1999. Recombinant adenovirus gene transfer in adults with partial ornithine transcarbamylase deficiency (OTCD). Hum. Gene Ther. 10:2419-2437. - PubMed
-
- Blair, G. E., S. C. Dixon, S. A. Griffiths, and M. E. Zajdel. 1989. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res. 14:339-346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

