Evidence suggesting that Pif1 helicase functions in DNA replication with the Dna2 helicase/nuclease and DNA polymerase delta
- PMID: 16537895
- PMCID: PMC1430326
- DOI: 10.1128/MCB.26.7.2490-2500.2006
Evidence suggesting that Pif1 helicase functions in DNA replication with the Dna2 helicase/nuclease and DNA polymerase delta
Abstract
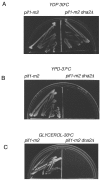
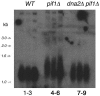
The precise machineries required for two aspects of eukaryotic DNA replication, Okazaki fragment processing (OFP) and telomere maintenance, are poorly understood. In this work, we present evidence that Saccharomyces cerevisiae Pif1 helicase plays a wider role in DNA replication than previously appreciated and that it likely functions in conjunction with Dna2 helicase/nuclease as a component of the OFP machinery. In addition, we show that Dna2, which is known to associate with telomeres in a cell-cycle-specific manner, may be a new component of the telomere replication apparatus. Specifically, we show that deletion of PIF1 suppresses the lethality of a DNA2-null mutant. The pif1delta dna2delta strain remains methylmethane sulfonate sensitive and temperature sensitive; however, these phenotypes can be suppressed by further deletion of a subunit of pol delta, POL32. Deletion of PIF1 also suppresses the cold-sensitive lethality and hydroxyurea sensitivity of the pol32delta strain. Dna2 is thought to function by cleaving long flaps that arise during OFP due to excessive strand displacement by pol delta and/or by an as yet unidentified helicase. Thus, suppression of dna2delta can be rationalized if deletion of POL32 and/or PIF1 results in a reduction in long flaps that require Dna2 for processing. We further show that deletion of DNA2 suppresses the long-telomere phenotype and the high rate of formation of gross chromosomal rearrangements in pif1Delta mutants, suggesting a role for Dna2 in telomere elongation in the absence of Pif1.
Figures




References
-
- Ayyagari, R., X. V. Gomes, D. A. Gordenin, and P. M. J. Burgers. 2003. Okazaki fragment maturation in yeast. I. Distribution of functions between Fen1 and Dna2. J. Biol. Chem. 278:1618-1625. - PubMed
-
- Bae, S. H., K.-H. Bae, J. A. Kim, and Y. S. Seo. 2001. RPA governs endonuclease switching during processing of Okazaki fragments in eukaryotes. Nature 412:456-461. - PubMed
-
- Bessler, J. B., J. Z. Torre, and V. A. Zakian. 2001. The Pif1p subfamily of helicases: region-specific DNA helicases? Trends Cell Biol. 11:60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
