Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene
- PMID: 16537897
- PMCID: PMC1430335
- DOI: 10.1128/MCB.26.7.2511-2518.2006
Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene
Abstract
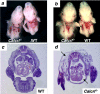
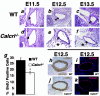
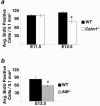
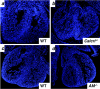
Adrenomedullin (AM) is a multifunctional peptide vasodilator that is essential for life. To date, numerous in vitro studies have suggested that AM can mediate its biological effects through at least three different receptors. To determine the in vivo importance of the most likely candidate receptor, calcitonin receptor-like receptor, a gene-targeted knockout model of the gene was generated. Mice heterozygous for the targeted Calcrl allele appear normal, survive to adulthood, and reproduce. However, heterozygote matings fail to produce viable Calcrl-/- pups, demonstrating that Calcrl is essential for survival. Timed matings confirmed that Calcrl-/- embryos die between embryonic day 13.5 (E13.5) and E14.5 of gestation. The Calcrl-/- embryos exhibit extreme hydrops fetalis and cardiovascular defects, including thin vascular smooth muscle walls and small, disorganized hearts remarkably similar to the previously characterized AM-/- phenotype. In vivo assays of cellular proliferation and apoptosis in the hearts and vasculature of Calcrl-/- and AM-/- embryos support the concept that AM signaling is a crucial mediator of cardiovascular development. The Calcrl gene targeted mice provide the first in vivo genetic evidence that CLR functions as an AM receptor during embryonic development.
Figures



Similar articles
-
Adrenomedullin signaling is necessary for murine lymphatic vascular development.J Clin Invest. 2008 Jan;118(1):40-50. doi: 10.1172/JCI33302. J Clin Invest. 2008. PMID: 18097475 Free PMC article.
-
Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene.Circulation. 2001 Oct 16;104(16):1964-71. doi: 10.1161/hc4101.097111. Circulation. 2001. PMID: 11602502
-
Shared and separate functions of the RAMP-based adrenomedullin receptors.Peptides. 2011 Jul;32(7):1540-50. doi: 10.1016/j.peptides.2011.05.022. Epub 2011 May 27. Peptides. 2011. PMID: 21645567 Review.
-
Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene.Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):615-9. doi: 10.1073/pnas.98.2.615. Epub 2001 Jan 9. Proc Natl Acad Sci U S A. 2001. PMID: 11149956 Free PMC article.
-
The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism.Bone. 2008 Nov;43(5):813-8. doi: 10.1016/j.bone.2008.07.003. Epub 2008 Jul 18. Bone. 2008. PMID: 18687416 Review.
Cited by
-
Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146.J Intern Med. 2017 Jan;281(1):25-40. doi: 10.1111/joim.12528. Epub 2016 Jun 16. J Intern Med. 2017. PMID: 27306986 Free PMC article. Review.
-
Adrenomedullin: Not Just Another Gastrointestinal Peptide.Biomolecules. 2022 Jan 18;12(2):156. doi: 10.3390/biom12020156. Biomolecules. 2022. PMID: 35204657 Free PMC article. Review.
-
A Focus on the Pathophysiology of Adrenomedullin Expression: Endothelitis and Organ Damage in Severe Viral and Bacterial Infections.Cells. 2024 May 22;13(11):892. doi: 10.3390/cells13110892. Cells. 2024. PMID: 38891025 Free PMC article. Review.
-
Probing the Mechanism of Receptor Activity-Modifying Protein Modulation of GPCR Ligand Selectivity through Rational Design of Potent Adrenomedullin and Calcitonin Gene-Related Peptide Antagonists.Mol Pharmacol. 2018 Apr;93(4):355-367. doi: 10.1124/mol.117.110916. Epub 2018 Jan 23. Mol Pharmacol. 2018. PMID: 29363552 Free PMC article.
-
MicroRNA 139-5p coordinates APLNR-CXCR4 crosstalk during vascular maturation.Nat Commun. 2016 Apr 12;7:11268. doi: 10.1038/ncomms11268. Nat Commun. 2016. PMID: 27068353 Free PMC article.
References
-
- Aiyar, N., K. Rand, N. A. Elshourbagy, Z. Zeng, J. E. Adamou, D. J. Bergsma, and Y. Li. 1996. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J. Biol. Chem. 271:11325-11329. - PubMed
-
- Albertin, G., M. Rucinski, G. Carraro, M. Forneris, P. Andreis, L. K. Malendowicz, and G. G. Nussdorfer. 2005. Adrenomedullin and vascular endothelium growth factor genes are overexpressed in the regenerating rat adrenal cortex, and AM and VEGF reciprocally enhance their mRNA expression in cultured rat adrenocortical cells. Int. J. Mol. Med. 16:431-435. - PubMed
-
- Allaker, R. P., and S. Kapas. 2003. Adrenomedullin and mucosal defence: interaction between host and microorganism. Regul. Pept. 112:147-152. - PubMed
-
- Autelitano, D. J. 1998. Cardiac expression of genes encoding putative adrenomedullin/calcitonin gene-related peptide receptors. Biochem. Biophys. Res. Commun. 250:689-693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
