Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation
- PMID: 16537911
- PMCID: PMC1430337
- DOI: 10.1128/MCB.26.7.2675-2687.2006
Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation
Abstract
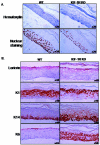
The insulin-like growth factor 1 receptor (IGF-1R) is a multifunctional receptor that mediates signals for cell proliferation, differentiation, and survival. Genetic experiments showed that IGF-1R inactivation in skin results in a disrupted epidermis. However, because IGF-1R-null mice die at birth, it is difficult to study the effects of IGF-1R on skin. By using a combined approach of conditional gene ablation and a three-dimensional organotypic model, we demonstrate that IGF-1R-deficient skin cocultures show abnormal maturation and differentiation patterns. Furthermore, IGF-1R-null keratinocytes exhibit accelerated differentiation and decreased proliferation. Investigating the signaling pathway downstream of IGF-1R reveals that insulin receptor substrate 2 (IRS-2) overexpression compensates for the lack of IGF-1R, whereas IRS-1 overexpression does not. We also demonstrate that phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1 and 2 are involved in the regulation of skin keratinocyte differentiation and take some part in mediating the inhibitory signal of IGF-1R on differentiation. In addition, we show that mammalian target of rapamycin plays a specific role in mediating IGF-1R impedance of action on keratinocyte differentiation. In conclusion, these results reveal that IGF-1R plays an inhibitory role in the regulation of skin development and differentiation.
Figures



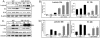


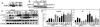
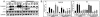
Similar articles
-
IRS-2 branch of IGF-1 receptor signaling is essential for appropriate timing of myelination.J Neurochem. 2008 Nov;107(4):907-17. doi: 10.1111/j.1471-4159.2008.05631.x. Epub 2008 Sep 18. J Neurochem. 2008. PMID: 18717815
-
Aberrant expression and activation of insulin-like growth factor-1 receptor (IGF-1R) are mediated by an induction of IGF-1R promoter activity and stabilization of IGF-1R mRNA and contributes to growth factor independence and increased survival of the pancreatic cancer cell line MIA PaCa-2.Oncogene. 2001 Dec 13;20(57):8203-14. doi: 10.1038/sj.onc.1205044. Oncogene. 2001. PMID: 11781836
-
Insulin receptor substrate 1 (IRS-1) plays a unique role in normal epidermal physiology.J Cell Physiol. 2007 Nov;213(2):519-27. doi: 10.1002/jcp.21131. J Cell Physiol. 2007. PMID: 17508357
-
Insulin-like growth factor-I receptor signal transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK-STAT) pathway.Biofactors. 2009 Jan-Feb;35(1):76-81. doi: 10.1002/biof.20. Biofactors. 2009. PMID: 19319849 Review.
-
Genetic blockade of the insulin-like growth factor 1 receptor for human malignancy.Novartis Found Symp. 2004;262:177-89; discussion 190-2, 265-8. Novartis Found Symp. 2004. PMID: 15562829 Review.
Cited by
-
PKCη promotes a proliferation to differentiation switch in keratinocytes via upregulation of p27Kip1 mRNA through suppression of JNK/c-Jun signaling under stress conditions.Cell Death Dis. 2011 May 19;2(5):e157. doi: 10.1038/cddis.2011.40. Cell Death Dis. 2011. PMID: 21593789 Free PMC article.
-
"One coin, two aspects": The role of IGF1R singling in chronic pain.Mol Pain. 2025 Jan-Dec;21:17448069251350856. doi: 10.1177/17448069251350856. Epub 2025 Jun 4. Mol Pain. 2025. PMID: 40464415 Free PMC article. No abstract available.
-
Cutaneous innervation in impaired diabetic wound healing.Transl Res. 2021 Oct;236:87-108. doi: 10.1016/j.trsl.2021.05.003. Epub 2021 May 23. Transl Res. 2021. PMID: 34029747 Free PMC article. Review.
-
A Case of Hyperandrogenism, Insulin Resistance, and Acanthosis Nigricans Syndrome; Increase in Proliferating Cell Nuclear Antigen and Decrease in Loricrin in Acanthosis Nigricans.Ann Dermatol. 2016 Oct;28(5):637-639. doi: 10.5021/ad.2016.28.5.637. Epub 2016 Sep 30. Ann Dermatol. 2016. PMID: 27746646 Free PMC article. No abstract available.
-
Effects of Composite Supplement Containing Collagen Peptide and Ornithine on Skin Conditions and Plasma IGF-1 Levels-A Randomized, Double-Blind, Placebo-Controlled Trial.Mar Drugs. 2018 Dec 3;16(12):482. doi: 10.3390/md16120482. Mar Drugs. 2018. PMID: 30513923 Free PMC article. Clinical Trial.
References
-
- Accili, D., J. Nakae, J. J. Kim, B. C. Park, and K. I. Rother. 1999. Targeted gene mutations define the roles of insulin and IGF-I receptors in mouse embryonic development. J. Pediatr. Endocrinol. Metab. 12:475-485. - PubMed
-
- Baker, J., J. P. Liu, E. J. Robertson, and A. Efstratiadis. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73-82. - PubMed
-
- Baserga, R., A. Hongo, M. Rubini, M. Prisco, and B. Valentinis. 1997. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim. Biophys. Acta 1332:F105-F126. - PubMed
-
- Calautti, E., J. Li, S. Saoncella, J. L. Brissette, and P. F. Goetinck. 2005. Phosphoinositide 3-kinase signaling to AKT promotes keratinocyte differentiation versus death. J. Biol. Chem. 280:32856-32865. - PubMed
-
- De Meyts, P., J. Palsgaard, W. Sajid, A. M. Theede, and H. Aladdin. 2004. Structural biology of insulin and IGF-1 receptors. Novartis Found. Symp. 262:160-171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
