Expression of Prox1 during mouse cochlear development
- PMID: 16538679
- PMCID: PMC2572724
- DOI: 10.1002/cne.20944
Expression of Prox1 during mouse cochlear development
Abstract
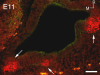
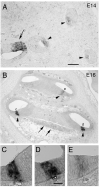
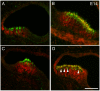
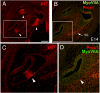
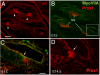
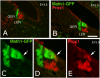
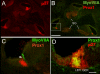
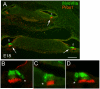
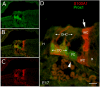
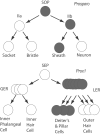
We carried out an analysis of the expression of Prox1, a homeo-domain transcription factor, during mouse inner ear development with particular emphasis on the auditory system. Prox1 is expressed in the otocyst beginning at embryonic day (E)11, in the developing vestibular sensory patches. Expression is down regulated in maturing (myosin VIIA immunoreactive) vestibular hair cells and subsequently in the underlying support cell layer by E16.5. In the auditory sensory epithelium, Prox1 is initially expressed at embryonic day 14.5 in a narrow stripe of cells at the base of the cochlea. This stripe encompasses the full thickness of the sensory epithelium, including developing hair cells and support cells. Over the next several days the stripe of expression extends to the apex, and as the sensory epithelium differentiates Prox1 becomes restricted to a subset of support cells. Double labeling for Prox1 and cell-type-specific markers revealed that the outer hair cells transiently express Prox1. After E18, Prox1 protein is no longer detectable in hair cells, but it continues to be expressed in support cells for the rest of embryogenesis and into the second postnatal week. During this time, Prox1 is not expressed in all support cell types in the organ of Corti, but is restricted to developing Deiters' and pillar cells. The expression is maintained in these cells into the second week of postnatal life, at which time Prox1 is dynamically down regulated. These studies form a baseline from which we can analyze the role of Prox1 in vertebrate sensory development.
J. Comp. Neurol. 496:172-186, 2006. (c) 2006 Wiley-Liss, Inc.
Figures


References
-
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense- organ development. Development. 1998;125:4645–4654. - PubMed
-
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. - PubMed
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
-
- Blanco J, Girard F, Kamachi Y, Kondoh H, Gehring WJ. Functional analysis of the chicken delta1-crystallin enhancer activity in Drosophila reveals remarkable evolutionary conservation between chicken and fly. Development. 2005;132:1895–1905. - PubMed
-
- Bryant J, Goodyear RJ, Richardson GP. Sensory organ development in the inner ear: molecular and cellular mechanisms. Br Med Bull. 2002;63:39–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

