Development of cyclic gamma-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities
- PMID: 16539382
- PMCID: PMC1484467
- DOI: 10.1021/jm0510326
Development of cyclic gamma-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities
Abstract
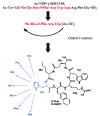
A series of cyclic lactam analogues of gamma-MSH (H-Tyr1-Val2-Met3-Gly4-His5-Phe6-Arg7-Trp8-Asp9-Arg10-Phe11-Gly12-OH) with a bulky hydrophobic residue in the direct proximity to the pharmacophore (Xaa-D-Phe/D-Nal(2')-Arg-Trp) were designed and synthesized by solid-phase methods. A variety of amino acids with a broad range of hydrophobic/hydrophilic properties was introduced in position 5 to further explore their complementary role in receptor selectivity. Biological evaluation of these peptides revealed several analogues with potent hMC3R agonist and hMC3R/hMC5R antagonist activities, and good receptor selectivity. Analogue 4, c[Nle-Arg-D-Phe-Arg-Trp-Glu]-NH2, was found to be a very potent and selective hMC3R agonist (EC50=1.2 nM, 112% act). In addition, analogue 13, c[Nle-Val-D-Nal(2')-Arg-Trp-Glu]-NH2, was identified as an hMC3R/hMC5R antagonist with the best selectivity against the hMC4R in this series (pA2(hMC3R)=8.4; pA2(hMC5R)=8.7). These results indicate the significance of steric factors in melanocortin receptor selectivity and suggest that introduction of bulky residues in the direct proximity to the melanocortin pharmacophore is an effective approach to design of novel hMC3R and hMC5R selective ligands.
Figures


Similar articles
-
Structure-activity relationships of cyclic lactam analogues of alpha-melanocyte-stimulating hormone (alpha-MSH) targeting the human melanocortin-3 receptor.J Med Chem. 2008 Jan 24;51(2):187-95. doi: 10.1021/jm070461w. Epub 2007 Dec 19. J Med Chem. 2008. PMID: 18088090 Free PMC article.
-
Structure-activity relationships of gamma-MSH analogues at the human melanocortin MC3, MC4, and MC5 receptors. Discovery of highly selective hMC3R, hMC4R, and hMC5R analogues.J Med Chem. 2003 Nov 6;46(23):4965-73. doi: 10.1021/jm030119t. J Med Chem. 2003. PMID: 14584947
-
Extensive structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5.J Pept Res. 2003 Nov;62(5):199-206. doi: 10.1034/j.1399-3011.2003.00087.x. J Pept Res. 2003. PMID: 14531843
-
Design of novel melanotropin agonists and antagonists with high potency and selectivity for human melanocortin receptors.Peptides. 2005 Aug;26(8):1481-5. doi: 10.1016/j.peptides.2005.03.020. Peptides. 2005. PMID: 15876475 Review.
-
Melanocortins and cardiovascular regulation.Eur J Pharmacol. 1998 Oct 30;360(1):1-14. doi: 10.1016/s0014-2999(98)00615-3. Eur J Pharmacol. 1998. PMID: 9845266 Review.
Cited by
-
Novel binding motif of ACTH analogues at the melanocortin receptors.Biochemistry. 2009 Oct 20;48(41):9775-84. doi: 10.1021/bi900634e. Biochemistry. 2009. PMID: 19743876 Free PMC article.
-
Structure-activity relationships of cyclic lactam analogues of alpha-melanocyte-stimulating hormone (alpha-MSH) targeting the human melanocortin-3 receptor.J Med Chem. 2008 Jan 24;51(2):187-95. doi: 10.1021/jm070461w. Epub 2007 Dec 19. J Med Chem. 2008. PMID: 18088090 Free PMC article.
-
Multiple N-methylation of MT-II backbone amide bonds leads to melanocortin receptor subtype hMC1R selectivity: pharmacological and conformational studies.J Am Chem Soc. 2010 Jun 16;132(23):8115-28. doi: 10.1021/ja101428m. J Am Chem Soc. 2010. PMID: 20496895 Free PMC article.
-
Approaches to the rational design of selective melanocortin receptor antagonists.Expert Opin Drug Discov. 2011 May;6(5):543-57. doi: 10.1517/17460441.2011.565743. Epub 2011 Mar 24. Expert Opin Drug Discov. 2011. PMID: 22646078 Free PMC article.
-
Effects of macrocycle size and rigidity on melanocortin receptor-1 and -5 selectivity in cyclic lactam alpha-melanocyte-stimulating hormone analogs.Chem Biol Drug Des. 2006 May;67(5):329-35. doi: 10.1111/j.1747-0285.2006.00383.x. Chem Biol Drug Des. 2006. PMID: 16784457 Free PMC article.
References
-
- Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signaling and obesity. J Endocrinol. 2002;172:411–421. - PubMed
-
- Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, de Vaux AE, Dym O, Castrucci AMF, Hintz MF, Riehem JP, Rao KR. α-Melanotropin: The minimal active sequence in the frog skin bioassay. J Med Chem. 1987;30:2126–2130. - PubMed
-
- Castrucci A-Mde L, Hadley ME, Sawyer TK, Wilkes BC, Al-Obeidi F, Staples DJ, de Vaux AE, Dym O, Hintz MF, Riehm JP, Rao RR, Hruby VJ. α-Melanotropin: The minimal active sequence in the lizard skin bioassay. Gen Comput Endocrinol. 1989;73:157–163. - PubMed
-
- Hadley, M. E.; Castrucci, A.-M. de L. Melanotropin Mechanisms of Action: Melanosome Movements. In The Melanotropic Peptides. Mechanisms of Action and Biomedical Applications; Hadley, M. E., Ed.; CRC Press: Boca Raton, FL, 1988; Vol. III, pp 15–25.
-
- Cone, R. D., Ed. The melanocortin system. Ann NY Acad Sci2003, 994, pp 1–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

