A long-term, open-label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with other disease-modifying antirheumatic drugs
- PMID: 16540554
- PMCID: PMC1798461
- DOI: 10.1136/ard.2005.038349
A long-term, open-label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with other disease-modifying antirheumatic drugs
Abstract
Objective: To evaluate the long-term safety and efficacy of etanercept in patients with rheumatoid arthritis.
Methods: 549 patients entered this 5-year, open-label extension study and received etanercept 25 mg twice weekly. All patients showed inadequate responses to disease-modifying antirheumatic drugs before entry into the double-blind studies. Safety assessments were carried out at regular intervals. Primary efficacy end points were the numbers of painful and swollen joints; secondary variables included American College of Rheumatology (ACR) response rate, Disease Activity Score and acute-phase reactants. Efficacy was analysed using the last-observation-carried-forward approach.
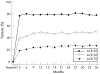
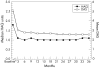
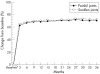
Results: Of the 549 patients enrolled in the open-label trial, 467 (85%), 414 (75%) and 371 (68%) completed 1, 2 and 3 years, respectively; 363 (66%) remained in the study at the time of this analysis. A total exposure of 1498 patient-years, including the double-blind study, was accrued. In the open-label trial, withdrawals for efficacy-related and safety-related reasons were 11% and 13%, respectively. Frequent adverse events included upper respiratory infections, flu syndrome, rash and injection-site reactions. Rates of serious infections and malignancies remained unchanged over the course of the study; there were no reports of patients with central demyelinating disease or serious blood dyscrasias. After 3 years, ACR20, ACR50 and ACR70 response rates were 78%, 51% and 27%, respectively. The Disease Activity Score score was reduced to 3.0 at 3 months and 2.6 at 3 years from 5.1. A sustained improvement was found in Health Assessment Questionnaire scores throughout the 3-year time period.
Conclusion: After 3 years of treatment, etanercept showed sustained efficacy and a favourable safety profile.
Conflict of interest statement
Competing interests: LK has received research grants and has been consultant and speaker on symposia arranged by Wyeth, Schering Plough, Abbott, Bristol Myers Squibb and Amgen. VR‐V has participated in clinical trials sponsored by Abbott, Schering Plough, Wyeth, and has given lectures paid by Schering Plough and Wyeth. MD has been paid by Wyeth for running educational programmes, and has received grants from Wyeth for conducting research. MG is a paid participating investigator and has given lectures for Wyeth Research. JW is an employee of Wyeth Research.
References
-
- Bathon J M, Martin R W, Fleischmann R M, Tesser J R, Schiff M H, Keystone E C.et al A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis [comment] [erratum appears in N Engl J Med 2001;344:240]. N Engl J Med 20003431586–1593. - PubMed
-
- Weinblatt M E, Kremer J M, Bankhurst A D, Bulpitt K J, Fleischmann R M, Fox R I.et al A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999340253–259. - PubMed
-
- Moreland L W, Schiff M H, Baumgartner S W, Tindall E A, Fleischmann R M, Bulpitt K J.et al Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999130478–486. - PubMed
-
- Genovese M C, Bathon J M, Martin R W, Fleischmann R M, Tesser J R, Schiff M H.et al Etanercept versus methotrexate in patients with early rheumatoid arthritis: two‐year radiographic and clinical outcomes. Arthritis Rheum 2002461443–1450. - PubMed
-
- Klareskog L, van der Heijde D, de Jager J P, Gough A, Kalden J, Malaise M.et al Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis. Lancet 2004363675–681. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical