Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling
- PMID: 16540575
- PMCID: PMC6673984
- DOI: 10.1523/JNEUROSCI.5453-05.2006
Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling
Abstract
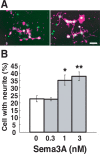
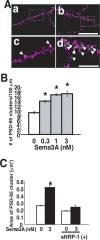
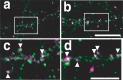
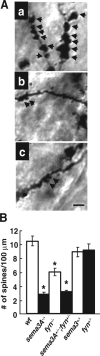
A member of semaphorin family, semaphorin3A (Sema3A), acts as a chemorepellent or chemoattractant on a wide variety of axons and dendrites in the development of the nervous systems. We here show that Sema3A induces clustering of both postsynaptic density-95 (PSD-95) and presynaptic synapsin I in cultured cortical neurons without changing the density of spines or filopodia. Neuropilin-1 (NRP-1), a receptor for Sema3A, is present on both axons and dendrites. When the cultured neurons are exposed to Sema3A, the cluster size of PSD-95 is markedly enhanced, and an extensive colocalization of PSD-95 and NRP-1 or actin-rich protrusion is seen. The effects of Sema3A on spine morphology are blocked by PP2, an Src type tyrosine kinase inhibitor, but not by the PP3, the inactive-related compound. In the cultured cortical neurons from fyn(-/-) mice, dendrites bear few spines, and Sema3A does not induce PSD-95 cluster formation on the dendrites. Sema3A and its receptor genes are highly expressed during the synaptogenic period of postnatal days 10 and 15. The cortical neurons in layer V, but not layer III, show a lowered density of synaptic bouton-like structure on dendrites in sema3A- and fyn-deficient mice. The neurons of the double-heterozygous mice show the lowered spine density, whereas those of single heterozygous mice show similar levels of the spine density as the wild type. These findings suggest that the Sema3A signaling pathway plays an important role in the regulation of dendritic spine maturation in the cerebral cortex neurons.
Figures




Similar articles
-
Regulation of spine development by semaphorin3A through cyclin-dependent kinase 5 phosphorylation of collapsin response mediator protein 1.J Neurosci. 2007 Nov 14;27(46):12546-54. doi: 10.1523/JNEUROSCI.3463-07.2007. J Neurosci. 2007. PMID: 18003833 Free PMC article.
-
Protein Tyrosine Phosphatase δ Mediates the Sema3A-Induced Cortical Basal Dendritic Arborization through the Activation of Fyn Tyrosine Kinase.J Neurosci. 2017 Jul 26;37(30):7125-7139. doi: 10.1523/JNEUROSCI.2519-16.2017. Epub 2017 Jun 21. J Neurosci. 2017. PMID: 28637841 Free PMC article.
-
Correlation between semaphorin3A-induced facilitation of axonal transport and local activation of a translation initiation factor eukaryotic translation initiation factor 4E.J Neurosci. 2004 Jul 7;24(27):6161-70. doi: 10.1523/JNEUROSCI.1476-04.2004. J Neurosci. 2004. PMID: 15240808 Free PMC article.
-
[Molecular mechanism of axon guidance].Nihon Shinkei Seishin Yakurigaku Zasshi. 2006 Jun;26(3):135-40. Nihon Shinkei Seishin Yakurigaku Zasshi. 2006. PMID: 16866215 Review. Japanese.
-
Semaphorin 3A: A new player in bone remodeling.Cell Adh Migr. 2014;8(1):5-10. doi: 10.4161/cam.27752. Epub 2013 Jan 1. Cell Adh Migr. 2014. PMID: 24589620 Free PMC article. Review.
Cited by
-
The transmembrane LRR protein DMA-1 promotes dendrite branching and growth in C. elegans.Nat Neurosci. 2011 Dec 4;15(1):57-63. doi: 10.1038/nn.2978. Nat Neurosci. 2011. PMID: 22138642 Free PMC article.
-
Activity-induced secretion of semaphorin 3A mediates learning.Eur J Neurosci. 2021 May;53(10):3279-3293. doi: 10.1111/ejn.15210. Epub 2021 Apr 5. Eur J Neurosci. 2021. PMID: 33772906 Free PMC article.
-
Fragile X Mental Retardation Protein is Involved in Protein Synthesis-Dependent Collapse of Growth Cones Induced by Semaphorin-3A.Front Neural Circuits. 2009 Sep 15;3:11. doi: 10.3389/neuro.04.011.2009. eCollection 2009. Front Neural Circuits. 2009. PMID: 19826618 Free PMC article.
-
Receptor protein tyrosine phosphatases control Purkinje neuron firing.Cell Cycle. 2020 Jan;19(2):153-159. doi: 10.1080/15384101.2019.1695995. Epub 2019 Dec 26. Cell Cycle. 2020. PMID: 31876231 Free PMC article.
-
Bilateral consequences of chronic unilateral deafferentation in the auditory system of the cricket Gryllus bimaculatus.Dev Neurosci. 2011;33(1):21-37. doi: 10.1159/000322887. Epub 2011 Feb 23. Dev Neurosci. 2011. PMID: 21346310 Free PMC article.
References
-
- Ahmari SE, Buchanan J, Smith SJ (2000). Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci 3:445–451. - PubMed
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I (2001). Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 4:367–373. - PubMed
-
- Campbell DS, Holt CE (2001). Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32:1013–1026. - PubMed
-
- Ethell IM, Irie F, Kalo MS, Couchman JR, Pasquale EB, Yamaguchi Y (2001). EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron 31:1001–1013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
