Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury
- PMID: 16540583
- PMCID: PMC6673975
- DOI: 10.1523/JNEUROSCI.5200-05.2006
Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury
Abstract
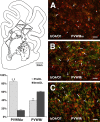
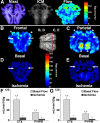
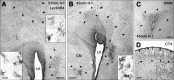
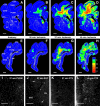
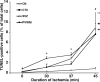
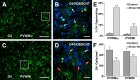
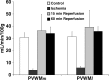
Although periventricular white matter injury (PWMI) is the leading cause of chronic neurological disability and cerebral palsy in survivors of premature birth, the cellular-molecular mechanisms by which ischemia-reperfusion contributes to the pathogenesis of PWMI are not well defined. To define pathophysiologic relationships among ischemia, acute cerebral white matter damage, and vulnerable target populations, we used a global cerebral ischemia-reperfusion model in the instrumented 0.65 gestation fetal sheep. We developed a novel method to make repeated measurements of cerebral blood flow using fluorescently labeled microspheres to resolve the spatial heterogeneity of flow in situ in three-dimensional space. Basal flow in the periventricular white matter (PVWM) was significantly lower than in the cerebral cortex. During global cerebral ischemia induced by carotid occlusion, flow to all regions was reduced by nearly 90%. Ischemia of 30 or 37 min duration generated selective graded injury to frontal and parietal PVWM, two regions of predilection for human PWMI. Injury was proportional to the duration of ischemia and increased markedly with 45 min of ischemia to extensively damage cortical and subcortical gray matter. Surprisingly, the distribution of PVWM damage was not uniform and not explained by heterogeneity in the degree of white matter ischemia. Rather, the extent of white matter damage coincided with the presence of a susceptible population of late oligodendrocyte progenitors. These data support that although ischemia is necessary to generate PWMI, the presence of susceptible populations of oligodendrocyte progenitors underlies regional predilection to injury.
Figures

References
-
- Back SA (2001). Recent advances in human perinatal white matter injury. In: Prog. brain res: glial cell function (Castellano-Lopez B, Nieto-Sampedro M, eds) pp. 131–147. Amsterdam: Elsevier. - PubMed
-
- Back SA, Rivkees S (2004). Emerging concepts in periventricular white matter injury. Semin Perinatol 28:405–414. - PubMed
-
- Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC (2002a). Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol 61:197–211. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
