Calpain 11 is unique to mouse spermatogenic cells
- PMID: 16541461
- PMCID: PMC1855627
- DOI: 10.1002/mrd.20466
Calpain 11 is unique to mouse spermatogenic cells
Abstract
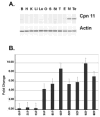
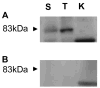
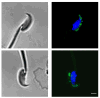
The calpains are a family of calcium-dependent thiol proteases involved in intracellular processing of proteins. They occur as heterodimers containing one of various large subunits and a common small subunit. Some of the large subunits are expressed ubiquitously and others are expressed in a restricted set of tissues. We have cloned the cDNA for mouse calpain 11 and demonstrated that it is expressed specifically in the mouse testis. The mRNA begins to accumulate in the testis between days 14 and 16 after birth, corresponding to the period of pachytene spermatocyte development. The protein is detected by day 18 after birth, during mid to late pachytene spermatocyte development, and is present in the acrosomal region of spermatozoa from the cauda epididymis. The expression of calpain 11 during spermatogenesis and its localization in spermatozoa suggest that it is involved in regulating calcium-dependent signal transduction events during meiosis and sperm functional processes.
Figures

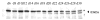
References
-
- Barnoy S, Glaser T, Kosower NS. The calpain-calpastatin system and protein degradation in fusing myoblasts. Biochim Biophys Acta. 1998;1402:52–60. - PubMed
-
- Barnoy S, Zipser Y, Glaser T, Grimberg Y, Kosower NS. Association of calpain (Ca2+-dependent thiol protease) with its endogenous inhibitor calpastatin in myoblasts. J Cell Biochem. 1999;74:522–31. - PubMed
-
- Ben-Aharon I, Brown PR, Etkovitz N, Eddy EM, Shalgi R. The expression of calpain 1 and calpain 2 in spermatogenic cells and spermatozoa of the mouse. Reproduction. 2005;129:435–442. - PubMed
-
- Carafoli E, Molinari M. Calpain: a protease in search of a function? Biochem Biophys Res Commun. 1998;247:193–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

