Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis
- PMID: 16542488
- PMCID: PMC1550886
- DOI: 10.1186/cc4851
Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis
Abstract
Introduction: The purpose of this study was to perform a systematic review and meta-analysis of exogenous surfactant administration to assess whether this therapy may be useful in adult patients with acute respiratory distress syndrome.
Methods: We performed a computerized literature search from 1966 to December 2005 to identify randomized clinical trials. The primary outcome measure was mortality 28-30 days after randomization. Secondary outcome measures included a change in oxygenation (PaO2:FiO2 ratio), the number of ventilation-free days, and the mean duration of ventilation. Meta-analysis was performed using the inverse variance method.
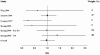
Results: Two hundred and fifty-one articles were identified. Five studies met our inclusion criteria. Treatment with pulmonary surfactant was not associated with reduced mortality compared with the control group (odds ratio 0.97; 95% confidence interval (CI) 0.73, 1.30). Subgroup analysis revealed no difference between surfactant containing surface protein or not - the pooled odds ratio for mortality was 0.87 (95% CI 0.48, 1.58) for trials using surface protein and the odds ratio was 1.08 (95% CI 0.72, 1.64) for trials without surface protein. The mean difference in change in the PaO2:FiO2 ratio was not significant (P = 0.11). There was a trend for improved oxygenation in the surfactant group (pooled mean change 13.18 mmHg, standard error 8.23 mmHg; 95% CI -2.95, 29.32). The number of ventilation-free days and the mean duration of ventilation could not undergo pooled analysis due to a lack of sufficient data.
Conclusion: Exogenous surfactant may improve oxygenation but has not been shown to improve mortality. Currently, exogenous surfactant cannot be considered an effective adjunctive therapy in acute respiratory distress syndrome.
Figures

Similar articles
-
Positioning for acute respiratory distress in hospitalised infants and children.Cochrane Database Syst Rev. 2022 Jun 6;6(6):CD003645. doi: 10.1002/14651858.CD003645.pub4. Cochrane Database Syst Rev. 2022. PMID: 35661343 Free PMC article.
-
Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome.Cochrane Database Syst Rev. 2007 Oct 17;2007(4):CD003063. doi: 10.1002/14651858.CD003063.pub3. Cochrane Database Syst Rev. 2007. PMID: 17943779 Free PMC article.
-
Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD006069. doi: 10.1002/14651858.CD006069.pub3. Cochrane Database Syst Rev. 2007. PMID: 17943881
-
Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome.Cochrane Database Syst Rev. 2004;(3):CD003063. doi: 10.1002/14651858.CD003063.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD003063. doi: 10.1002/14651858.CD003063.pub3. PMID: 15266470 Updated.
-
Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD006069. doi: 10.1002/14651858.CD006069.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD006069. doi: 10.1002/14651858.CD006069.pub3. PMID: 17636826 Updated.
Cited by
-
The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections.Ann Am Thorac Soc. 2015 May;12(5):765-74. doi: 10.1513/AnnalsATS.201411-507FR. Ann Am Thorac Soc. 2015. PMID: 25742123 Free PMC article. Review.
-
Computed tomography assessment of exogenous surfactant-induced lung reaeration in patients with acute lung injury.Crit Care. 2010;14(4):R135. doi: 10.1186/cc9186. Epub 2010 Jul 15. Crit Care. 2010. PMID: 20633284 Free PMC article. Clinical Trial.
-
Life-saving effect of pulmonary surfactant in premature babies.J Clin Invest. 2024 May 1;134(9):e179948. doi: 10.1172/JCI179948. J Clin Invest. 2024. PMID: 38690742 Free PMC article.
-
Rediscovering the Schulze-Hardy rule in competitive adsorption to an air-water interface.Langmuir. 2009 Sep 1;25(17):10045-50. doi: 10.1021/la9009724. Langmuir. 2009. PMID: 19705897 Free PMC article.
-
Exogenous surfactant may improve oxygenation but not mortality in adult patients with acute lung injury/acute respiratory distress syndrome: a meta-analysis of 9 clinical trials.J Cardiothorac Vasc Anesth. 2012 Oct;26(5):849-56. doi: 10.1053/j.jvca.2011.11.006. Epub 2012 Jan 20. J Cardiothorac Vasc Anesth. 2012. PMID: 22265270 Free PMC article.
References
-
- National Heart and Lung Institute . Task force on problems, research approaches, needs: the lung program Publication No (NIII) 73-432. Washington, DC: Department of Health, Education, and Welfare; 1972. pp. 165–180.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

