The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database
- PMID: 16542492
- PMCID: PMC1550902
- DOI: 10.1186/cc4854
The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database
Abstract
Introduction: To evaluate the impact of recent evidence-based treatments for severe sepsis in routine clinical care requires an understanding of the underlying epidemiology, particularly with regard to trends over time. We interrogated a high quality clinical database to examine trends in the incidence and mortality of severe sepsis over a nine-year period.
Methods: Admissions with severe sepsis occurring at any time within 24 hours of admission to critical care were identified to an established methodology using raw physiological data from the Intensive Care National Audit & Research Centre (ICNARC) Case Mix Programme Database, containing data from 343,860 admissions to 172 adult, general critical care units in England, Wales and Northern Ireland between December 1995 and January 2005. Generalised linear models were used to assess changes in the incidence, case mix, outcomes and activity of these admissions.
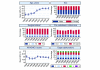
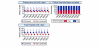
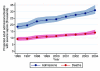
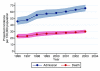
Results: In total, 92,672 admissions (27.0%) were identified as having severe sepsis in the first 24 hours following admission. The percentage of admissions with severe sepsis during the first 24 hours rose from 23.5% in 1996 to 28.7% in 2004. This represents an increase from an estimated 18,500 to 31,000 admissions to all 240 adult, general critical care units in England, Wales and Northern Ireland. Hospital mortality for admissions with severe sepsis decreased from 48.3% in 1996 to 44.7% in 2004, but the total number of deaths increased from an estimated 9,000 to 14,000. The treated incidence of severe sepsis per 100,000 population rose from 46 in 1996 to 66 in 2003, with the associated number of hospital deaths per 100,000 population rising from 23 to 30.
Conclusion: The population incidence of critical care admission with severe sepsis during the first 24 hours and associated hospital deaths are increasing. These baseline data provide essential information to those wishing to evaluate the introduction of the Surviving Sepsis Campaign care bundles in UK hospitals.
Figures


References
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. - PubMed
-
- Laterre PF, Levy H, Clermont G, Ball DE, Garg R, Nelson DR, Dhainaut JF, Angus DC. Hospital mortality and resource use in subgroups of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial. Crit Care Med. 2004;32:2207–2218. - PubMed
-
- Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–941. doi: 10.1001/jama.273.12.934. - DOI - PubMed
-
- Abraham E, Laterre PF, Garbino J, Pingleton S, Butler T, Dugernier T, Margolis B, Kudsk K, Zimmerli W, Anderson P, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29:503–510. doi: 10.1097/00003246-200103000-00006. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

