Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line
- PMID: 16543264
- PMCID: PMC1779734
- DOI: 10.1113/jphysiol.2006.106906
Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line
Abstract
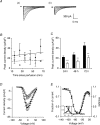
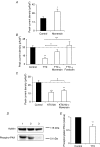
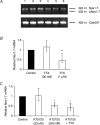
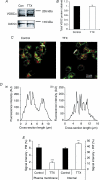
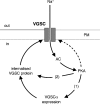
We have shown previously that voltage-gated Na(+) channels (VGSCs) are up-regulated in human metastatic disease (prostate, breast and small-cell lung cancers), and that VGSC activity potentiates metastatic cell behaviours. However, the mechanism(s) regulating functional VGSC expression in cancer cells remains unknown. We investigated the possibility of activity-dependent (auto)regulation of VGSC functional expression in the strongly metastatic Mat-LyLu model of rat prostate cancer. Pretreatment with tetrodotoxin (TTX) for 24-72 h subsequently suppressed peak VGSC current density without affecting voltage dependence. The hypothesis was tested that the VGSC auto-regulation occurred via VGSC-mediated Na(+) influx and subsequent activation of protein kinase A (PKA). Indeed, TTX pretreatment reduced the level of phosphorylated PKA, and the PKA inhibitor KT5720 decreased, whilst the adenylate cyclase activator forskolin and the Na(+) ionophore monensin both increased the peak VGSC current density. TTX reduced the mRNA level of Nav1.7, predominant in these cells, and VGSC protein expression at the plasma membrane, although the total VGSC protein level remained unchanged. TTX pretreatment eliminated the VGSC-dependent component of the cells' migration in Transwell assays. We concluded that the VGSC activity in Mat-LyLu rat prostate cancer cells was up-regulated in steady-state via a positive feedback mechanism involving PKA, and this enhanced the cells' migratory potential.
Figures

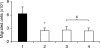
References
-
- Abdul M, Hoosein N. Inhibition by anticonvulsants of prostate-specific antigen and interleukin-6 secretion by human prostate cancer cells. Anticancer Res. 2001;21:2045–2048. - PubMed
-
- Abdul M, Hoosein N. Voltage-gated sodium ion channels in prostate cancer: expression and activity. Anticancer Res. 2002;22:1727–1730. - PubMed
-
- Allen DH, Lepple-Wienhues A, Cahalan MD. Ion channel phenotype of melanoma cell lines. J Membr Biol. 1997;155:27–34. - PubMed
-
- Anderson JD, Hansen TP, Lenkowski PW, Walls AM, Choudhury IM, Schenck HA, Friehling M, Holl GM, Patel MK, Sikes RA, Brown ML. Voltage-gated sodium channel blockers as cytostatic inhibitors of the androgen-independent prostate cancer cell line PC-3. Mol Cancer Ther. 2003;2:1149–1154. - PubMed
-
- Ben Fredj N, Grange J, Sadoul R, Richard S, Goldberg Y, Boyer V. Depolarization-induced translocation of the RNA-binding protein Sam68 to the dendrites of hippocampal neurons. J Cell Sci. 2004;117:1079–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

