COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model
- PMID: 16543381
- PMCID: PMC1458775
- DOI: 10.1073/pnas.0506352103
COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model
Erratum in
- Proc Natl Acad Sci U S A. 2006 Jun 27;103(26):10146
Abstract
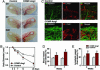
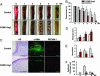
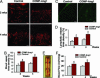
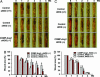
Microvascular dysfunction is a major cause of impaired wound healing seen in diabetic patients. Therefore, reestablishment of structural and functional microvasculature could be beneficial to promote wound healing in these patients. Angiopoietin-1 (Ang1) is a specific growth factor functioning to generate a stable and functional vasculature through the Tie2 and Tie1 receptors. Here we determined the effectiveness of cartilage oligomeric matrix protein (COMP)-Ang1, a soluble, stable, and potent form of Ang1, on promotion of healing in cutaneous wounds of diabetic mice. An excisional full-thickness wound was made in the dorsal side of the tail of diabetic (db/db) mice, and mice were then treated systemically with adenovirus (Ade) encoding COMP-Ang1 or with control virus encoding beta-gal (Ade-beta-gal) or treated topically with recombinant COMP-Ang1 protein or BSA. Time course observations revealed that mice treated with Ade-COMP-Ang1 or COMP-Ang1 protein showed accelerated wound closure and epidermal and dermal regeneration, enhanced angiogenesis and lymphangiogenesis, and higher blood flow in the wound region compared with mice treated with control virus or BSA. COMP-Ang1 promotion of wound closure and angiogenesis was not dependent on endothelial nitric oxide synthase or inducible nitric oxide synthase alone. Taken together, these findings indicate that COMP-Ang1 can promote wound healing in diabetes through enhanced angiogenesis, lymphangiogenesis, and blood flow.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
-
- Martin P. Science. 1997;276:75–81. - PubMed
-
- Tonnesen M. G., Feng X., Clark R. A. J. Invest. Dermatol. Symp. Proc. 2000;5:40–46. - PubMed
-
- Hirakawa S., Detmar M. J. Dermatol. Sci. 2004;35:1–8. - PubMed
-
- Yancopoulos G. D., Davis S., Gale N. W., Rudge J. S., Wiegand S. J., Holash J. Nature. 2000;407:242–248. - PubMed
-
- Tammela T., Petrova T. V., Alitalo K. Trends Cell Biol. 2005;15:434–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

