CYP707A1 and CYP707A2, which encode abscisic acid 8'-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis
- PMID: 16543410
- PMCID: PMC1459320
- DOI: 10.1104/pp.106.079475
CYP707A1 and CYP707A2, which encode abscisic acid 8'-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis
Abstract
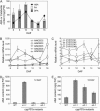
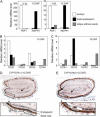
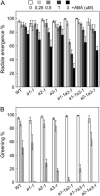
Endogenous abscisic acid (ABA) levels are regulated by both biosynthesis and catabolism of the hormone. ABA 8'-hydroxylase is considered to be the key catabolic enzyme in many physiological processes. We have previously identified that four members of the Arabidopsis (Arabidopsis thaliana) CYP707A gene family (CYP707A1 to CYP707A4) encode ABA 8'-hydroxylases, and that the cyp707a2 mutants showed an increase in ABA levels in dry and imbibed seeds. In this study, we showed that the cyp707a1 mutant accumulated ABA to higher levels in dry seeds than the cyp707a2 mutant. Expression analysis showed that the CYP707A1 was expressed predominantly during mid-maturation and was down-regulated during late-maturation. Concomitantly, the CYP707A2 transcript levels increased from late-maturation to mature dry seed. Phenotypic analysis of single and double cyp707a mutants indicates that the CYP707A1 is important for reducing ABA levels during mid-maturation. On the other hand, CYP707A2 is responsible for the regulation of ABA levels from late-maturation to germination. Moreover, CYP707A1 and CYP707A3 were also shown to be involved in postgermination growth. Spatial expression analysis suggests that CYP707A1 was expressed predominantly in embryo during mid-maturation, whereas CYP707A2 expression was detected in both embryo and endosperm from late-maturation to germination. Our results demonstrate that each CYP707A gene plays a distinct role during seed development and postgermination growth.
Figures



References
-
- Abe M, Takahashi T, Komeda Y (1999) Cloning and characterization of an L1 layer-specific gene in Arabidopsis thaliana. Plant Cell Physiol 40: 571–580 - PubMed
-
- Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, Jullien M (2004) Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219: 479–488 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen HM, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Asami T, Sekimata K, Wang JM, Yoneyama K, Takeuchi Y, Yoshida S (1999) Preparation of (±)-[1,2-13C2] abscisic acid for use as a stable and pure internal standard. J Chem Res (Synop) 11: 658–659
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

