Intestinal ABCA1 directly contributes to HDL biogenesis in vivo
- PMID: 16543947
- PMCID: PMC1401485
- DOI: 10.1172/JCI27352
Intestinal ABCA1 directly contributes to HDL biogenesis in vivo
Abstract
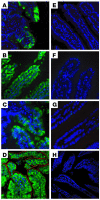
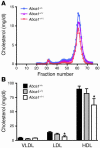
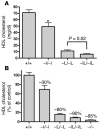
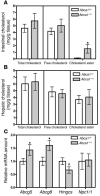
Plasma HDL cholesterol levels are inversely related to risk for atherosclerosis. The ATP-binding cassette, subfamily A, member 1 (ABCA1) mediates the rate-controlling step in HDL particle formation, the assembly of free cholesterol and phospholipids with apoA-I. ABCA1 is expressed in many tissues; however, the physiological functions of ABCA1 in specific tissues and organs are still elusive. The liver is known to be the major source of plasma HDL, but it is likely that there are other important sites of HDL biogenesis. To assess the contribution of intestinal ABCA1 to plasma HDL levels in vivo, we generated mice that specifically lack ABCA1 in the intestine. Our results indicate that approximately 30% of the steady-state plasma HDL pool is contributed by intestinal ABCA1 in mice. In addition, our data suggest that HDL derived from intestinal ABCA1 is secreted directly into the circulation and that HDL in lymph is predominantly derived from the plasma compartment. These data establish a critical role for intestinal ABCA1 in plasma HDL biogenesis in vivo.
Figures



References
-
- Glomset J.A. The plasma lecithin: cholesterol acyltransferase reaction. J. Lipid Res. 1968;9:155–167. - PubMed
-
- Glomset J.A., Norum K.R. The metabolic role of lecithin: cholesterol acyltransferase. Perspectives from pathology. Adv. Lipid Res. 1973;11:1–65. - PubMed
-
- Linsel-Nitschke P., Tall A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 2005;4:193–205. - PubMed
-
- Schmitz G., Langmann T. Structure, function and regulation of the ABC1 gene product. Curr. Opin. Lipidol. 2001;12:129–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

