Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance
- PMID: 16543948
- PMCID: PMC1401484
- DOI: 10.1172/JCI27203
Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance
Abstract
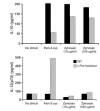
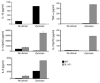
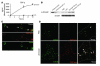
Emerging evidence suggests critical roles for APCs in suppressing immune responses. Here, we show that zymosan, a stimulus for TLR2 and dectin-1, regulates cytokine secretion in DCs and macrophages to induce immunological tolerance. First, zymosan induces DCs to secrete abundant IL-10 but little IL-6 and IL-12(p70). Induction of IL-10 is dependent on TLR2- and dectin-1-mediated activation of ERK MAPK via a mechanism independent of the activation protein 1 (AP-1) transcription factor c-Fos. Such DCs stimulate antigen-specific CD4+ T cells poorly due to IL-10 and the lack of IL-6. Second, zymosan induces F4-80+ macrophages in the splenic red pulp to secrete TGF-beta. Consistent with these effects on APCs, injection of zymosan plus OVA into mice results in OVA-specific T cells that secrete little or no Th1 or Th2 cytokines, but secrete robust levels of IL-10, and are unresponsive to challenge with OVA plus adjuvant. Finally, coinjection of zymosan with OVA plus LPS suppresses the response to OVA via a mechanism dependent on IL-10, TGF-beta, and lack of IL-6. Together, our data demonstrate that zymosan stimulates IL-10+ IL-12(p70)- IL-6low regulatory DCs and TGF-beta+ macrophages to induce immunological tolerance. These data suggest several targets for pharmacological modulation of immune responses in various clinical settings.
Figures



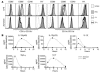



References
-
- Janeway C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. - PubMed
-
- Shortman K., Liu Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002;2:151–161. - PubMed
-
- Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 2004;199:227–250. - PubMed
-
- Pasare C., Medzhitov R. Toll-like receptors and acquired immunity. Semin. Immunol. 2004;16:23–26. - PubMed
-
- Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

