Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization
- PMID: 16543952
- PMCID: PMC1401486
- DOI: 10.1172/JCI27414
Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization
Abstract
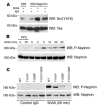
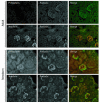
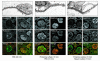
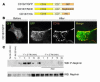
A properly established and maintained podocyte intercellular junction, or slit diaphragm, is a necessary component of the selective permeability barrier of the kidney glomerulus. The observation that mutation or deletion of the slit diaphragm transmembrane protein nephrin results in failure of podocyte foot process morphogenesis and concomitant proteinuria first suggested the hypothesis that nephrin serves as a component of a signaling complex that directly integrates podocyte junctional integrity with cytoskeletal dynamics. The observations made herein provide the first direct evidence to our knowledge for a phosphorylation-mediated signaling mechanism by which this integrative function is derived. Our data support the model that during podocyte intercellular junction formation, engagement of the nephrin ectodomain induces transient Fyn catalytic activity that results in nephrin phosphorylation on specific nephrin cytoplasmic domain tyrosine residues. We found that this nephrin phosphorylation event resulted in recruitment of the SH2-SH3 domain-containing adapter protein Nck and assembly of actin filaments in an Nck-dependent fashion. Considered in the context of the role of nephrin family proteins in other organisms and the integral relationship of actin dynamics and junction formation, these observations establish a function for nephrin in regulating actin cytoskeletal dynamics.
Figures







Comment in
-
Dynamics at the slit diaphragm--is nephrin actin'?Nephrol Dial Transplant. 2007 Jan;22(1):37-9. doi: 10.1093/ndt/gfl485. Epub 2006 Sep 2. Nephrol Dial Transplant. 2007. PMID: 16951421 No abstract available.
References
-
- Johnstone D., Holzman L.B. Clinical impact of research on the podocyte slit diaphragm. Nature Clinical Practice Nephrology. 2006 In press. - PubMed
-
- Kestila M., et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol. Cell. 1998;1:575–582. - PubMed
-
- Putaala H., Soininen R., Kilpelainen P., Wartiovaara J., Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum. Mol. Genet. 2001;10:1–8. - PubMed
-
- Hamano Y., et al. Determinants of vascular permeability in the kidney glomerulus. J. Biol. Chem. 2002;277:31154–31162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

