siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene
- PMID: 16545478
- PMCID: PMC7114600
- DOI: 10.1016/j.jconrel.2006.01.022
siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene
Abstract
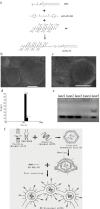
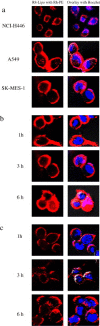
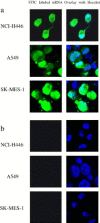
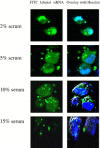
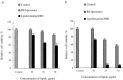
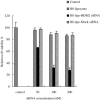
Development of RNA interference (RNAi) technology utilizing the short interfering RNA sequences (siRNA) based 'targeted' therapeutics has focused on creating methods for delivering siRNAs to cells and for enhancing siRNA stability in vitro and in vivo. Here, we describe a novel approach for siRNA cellular delivery using siRNA encapsulated into liposomes additionally bearing arginine octamer (R8) molecules attached to their outer surface (R8-liposomes). The R8-liposomal human double minute gene 2 (HDM2)-siRNA demonstrated a significant stability against degradation in the blood serum (siRNA-loaded R8-liposomes remained intact even after 24-h incubation), and higher transfection efficiency into all three tested lung tumor cell lines. siRNA delivery successfully proceeds in the presence of plasma proteins, and R8-liposomes demonstrate low non-specific toxicity. The mechanism of action of R8-liposome-encapsulated siRNA is associated with the RNAi-mediated degradation of the target mRNA. siRNA in R8-liposomes effectively inhibited the targeted gene and significantly reduced the proliferation of cancer cells. The approach offers the potential for siRNA delivery for various in vitro and in vivo applications.
Figures


Similar articles
-
[Cell penetrating peptides enhance intracellular translocation and function of siRNA encapsulated in Pegylated liposomes].Yao Xue Xue Bao. 2006 Feb;41(2):142-8. Yao Xue Xue Bao. 2006. PMID: 16671545 Chinese.
-
The enhancement of gene silencing efficiency with chitosan-coated liposome formulations of siRNAs targeting HIF-1α and VEGF.Int J Pharm. 2015 Jan 15;478(1):147-154. doi: 10.1016/j.ijpharm.2014.10.065. Epub 2014 Nov 13. Int J Pharm. 2015. PMID: 25445537
-
Therapeutic targeting of siRNA/anti-cancer drug delivery system for non-melanoma skin cancer. Part I: Development and gene silencing of JAK1siRNA/5-FU loaded liposome nanocomplexes.Eur J Pharm Biopharm. 2024 Oct;203:114432. doi: 10.1016/j.ejpb.2024.114432. Epub 2024 Aug 2. Eur J Pharm Biopharm. 2024. PMID: 39097115
-
Strategies for in vivo delivery of siRNAs: recent progress.BioDrugs. 2010 Jun;24(3):195-205. doi: 10.2165/11534450-000000000-00000. BioDrugs. 2010. PMID: 20462284 Review.
-
Delivery strategies for siRNA-mediated gene silencing.Curr Drug Deliv. 2006 Apr;3(2):147-5. doi: 10.2174/156720106776359159. Curr Drug Deliv. 2006. PMID: 16611001 Review.
Cited by
-
Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting.RNA. 2007 Apr;13(4):431-56. doi: 10.1261/rna.459807. Epub 2007 Feb 28. RNA. 2007. PMID: 17329355 Free PMC article. Review.
-
Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment.Genes (Basel). 2023 Jun 28;14(7):1370. doi: 10.3390/genes14071370. Genes (Basel). 2023. PMID: 37510275 Free PMC article. Review.
-
Inhibition of pulmonary cancer progression by epidermal growth factor receptor-targeted transfection with Bcl-2 and survivin siRNAs.Cancer Gene Ther. 2015 Jul;22(7):335-43. doi: 10.1038/cgt.2015.18. Epub 2015 Apr 10. Cancer Gene Ther. 2015. PMID: 25857361
-
Delivery of antisense oligonucleotide using polyethylenimine-based lipid nanoparticle modified with cell penetrating peptide.Drug Deliv. 2019 Dec;26(1):965-974. doi: 10.1080/10717544.2019.1667453. Drug Deliv. 2019. PMID: 31544540 Free PMC article.
-
RNAi in Spodoptera frugiperda Sf9 Cells via Nanomaterial Mediated Delivery of dsRNA: A Comparison of Poly-l-arginine Polyplexes and Poly-l-arginine-Functionalized Au Nanoparticles.ACS Appl Mater Interfaces. 2020 Jun 10;12(23):25645-25657. doi: 10.1021/acsami.0c06234. Epub 2020 May 28. ACS Appl Mater Interfaces. 2020. PMID: 32412742 Free PMC article.
References
-
- Novina C.D., Sharp P.A. The RNAi revolution. Nature. 2004;430:161–164. - PubMed
-
- Chiu Y.L., Ali A., Chu C.Y., Cao H., Rana T.M. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem. Biol. 2004;11:1165–1175. - PubMed
-
- Soutschek J., Akinc A., Bramlage B., Charisse K., Constien R., Donoghue M., Elbashir S., Geick A., Hadwiger P., Harborth J., John M., Kesavan V., Lavine G., Pandey R.K., Racie T., Rajeev K.G., Rohl I., Toudjarska I., Wang G., Wuschko S., Bumcrot D., Koteliansky V., Limmer S., Manoharan M., Vornlocher H.P. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–1788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

