Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunisations
- PMID: 16545511
- PMCID: PMC7115539
- DOI: 10.1016/j.vaccine.2006.02.040
Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunisations
Abstract
A putative protective protein from Plasmodium falciparum merozoites, MSA2, was expressed in two different ways on the cell surface of the Gram-positive food-grade bacterium, Lactococcus lactis. The first display format exploits an LPXTG-type anchoring motif of the lactococcal proteinase PrtP to covalently anchor MSA2 to the genetically modified producer cells. In a second display format, MSA2 was fused to the peptidoglycan-binding domain (Protein Anchor) of the lactococcal cell wall hydrolase AcmA and was non-covalently rebound to the surface of non-genetically modified, non-living high-binder L. lactis cells, termed Gram-positive enhancer matrix (GEM) particles. The L. lactis recombinants carrying covalently bound MSA2 were used to immunise rabbits through nasal and oral routes. The highest levels of IgG antibodies reacting with near-native MSA2 on merozoites was elicited by oral administration. Intestinal antibodies to MSA2 were produced only after oral immunisation. MSA2-specific T(h)-cell activation could be demonstrated. Based on these results, the immunogenicity in oral immunisations of MSA2, bound non-covalently to non-genetically modified L. lactis GEM particles, was compared with MSA2 that was bound covalently to genetically modified L. lactis. These two forms elicited similar titres of serum antibodies. The results illustrate the potential of using non-genetically modified L. lactis as a safe vaccine delivery vehicle to elicit systemic antibodies, thereby avoiding the dissemination of recombinant DNA into the environment.
Figures

 GPI anchor sequence of MSA2. The arrow represents the nisin-inducible promoter of L. lactis. The lollypop represents a lactococcal transcriptional terminator sequence.
GPI anchor sequence of MSA2. The arrow represents the nisin-inducible promoter of L. lactis. The lollypop represents a lactococcal transcriptional terminator sequence.  signal sequence of PrtP.
signal sequence of PrtP.  prosequence of PrtP.
prosequence of PrtP.  one repeat of the AcmA cell wall anchor. The three repeats constitute the entire cell-wall anchor domain (PA).
one repeat of the AcmA cell wall anchor. The three repeats constitute the entire cell-wall anchor domain (PA).  cell wall anchor of PrtP (cP).
cell wall anchor of PrtP (cP).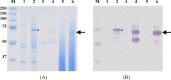
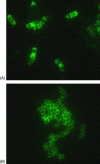
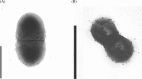

Similar articles
-
Age-dependent systemic antibody responses and immunisation-associated changes in mice orally and nasally immunised with Lactococcus lactis expressing a malaria parasite protein.Vaccine. 2009 Aug 6;27(36):4947-52. doi: 10.1016/j.vaccine.2009.06.011. Epub 2009 Jun 21. Vaccine. 2009. PMID: 19545652
-
Mucosal immunisation of mice with malaria protein on lactic acid bacterial cell walls.Vaccine. 2007 May 4;25(18):3636-45. doi: 10.1016/j.vaccine.2007.01.070. Epub 2007 Jan 22. Vaccine. 2007. PMID: 17280749
-
A Plasmodium falciparum GLURP-MSP3 chimeric protein; expression in Lactococcus lactis, immunogenicity and induction of biologically active antibodies.Vaccine. 2004 Mar 12;22(9-10):1188-98. doi: 10.1016/j.vaccine.2003.09.017. Vaccine. 2004. PMID: 15003647
-
Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum.Hum Vaccin. 2010 Jan;6(1):39-53. doi: 10.4161/hv.6.1.10712. Epub 2010 Jan 19. Hum Vaccin. 2010. PMID: 20061790 Review.
-
Lactic acid bacteria as live vaccines.Curr Issues Mol Biol. 2000 Jan;2(1):17-25. Curr Issues Mol Biol. 2000. PMID: 11464916 Review.
Cited by
-
Activated human nasal epithelial cells modulate specific antibody response against bacterial or viral antigens.PLoS One. 2013;8(2):e55472. doi: 10.1371/journal.pone.0055472. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405156 Free PMC article.
-
Protection against avian coronavirus conferred by oral vaccination with live bacteria secreting LTB-fused viral proteins.Vaccine. 2022 Jan 31;40(5):726-733. doi: 10.1016/j.vaccine.2021.12.053. Epub 2021 Dec 30. Vaccine. 2022. PMID: 34998606 Free PMC article.
-
System using tandem repeats of the cA peptidoglycan-binding domain from Lactococcus lactis for display of both N- and C-terminal fusions on cell surfaces of lactic acid bacteria.Appl Environ Microbiol. 2008 Feb;74(4):1117-23. doi: 10.1128/AEM.02012-07. Epub 2007 Dec 21. Appl Environ Microbiol. 2008. PMID: 18156338 Free PMC article.
-
Passive inhalation of dry powder influenza vaccine formulations completely protects chickens against H5N1 lethal viral challenge.Eur J Pharm Biopharm. 2018 Dec;133:85-95. doi: 10.1016/j.ejpb.2018.10.008. Epub 2018 Oct 9. Eur J Pharm Biopharm. 2018. PMID: 30312742 Free PMC article.
-
Innate and Adaptive Immune Responses in the Upper Respiratory Tract and the Infectivity of SARS-CoV-2.Viruses. 2022 Apr 29;14(5):933. doi: 10.3390/v14050933. Viruses. 2022. PMID: 35632675 Free PMC article. Review.
References
-
- Weiner H.L., Friedman A., Miller A., Khoury S.J., Al-Sabbagh A., Santos L. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Ann Rev Immunol. 1994;12:809–837. - PubMed
-
- Davis S.S. Nasal vaccines. Adv Drug Deliv Rev. 2001;51:21–42. - PubMed
-
- Norton P.M., Brown H.W.G., Le Page R.W.F. The immune response to Lactococcus lactis: implications for its use as a vaccine delivery vehicle. FEMS Microbiol Lett. 1994;1120:249–256. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

