Phenotypic characterization of Streptococcus pneumoniae biofilm development
- PMID: 16547018
- PMCID: PMC1428403
- DOI: 10.1128/JB.188.7.2325-2335.2006
Phenotypic characterization of Streptococcus pneumoniae biofilm development
Abstract
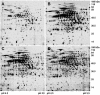
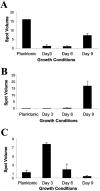
Streptococcus pneumoniae is among the most common pathogens associated with chronic otitis media with effusion, which has been hypothesized to be a biofilm disease. S. pneumoniae has been shown to form biofilms, however, little is known about the developmental process, the architecture, and the changes that occur upon biofilm development. In the current study we made use of a continuous-culture biofilm system to characterize biofilm development of 14 different S. pneumoniae strains representing at least 10 unique serotypes. The biofilm development process was found to occur in three distinct stages, including initial attachment, cluster formation, and biofilm maturation. While all 14 pneumococcal strains displayed similar developmental stages, the mature biofilm architecture differed significantly among the serotypes tested. Overall, three biofilm architectural groups were detected based on biomass, biofilm thickness, and cluster size. The biofilm viable cell counts and total protein concentration increased steadily over the course of biofilm development, reaching approximately 8 x 10(8) cells and approximately 15 mg of protein per biofilm after 9 days of biofilm growth. Proteomic analysis confirmed the presence of distinct biofilm developmental stages by the detection of multiple phenotypes over the course of biofilm development. The biofilm development process was found to correlate not only with differential production of proteins but also with a dramatic increase in the number of detectable proteins, indicating that biofilm formation by S. pneumoniae may be a far more complex process than previously anticipated. Protein identification revealed that proteins involved in virulence, adhesion, and resistance were more abundant under biofilm growth conditions. A possible role of the identified proteins in biofilm formation is discussed.
Figures



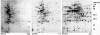
References
-
- Applegate, D. H., and J. D. Bryers. 1991. Effects on carbon and oxygen limitations and calcium concentrations on biofilm removal processes. Biotechnol. Bioeng. 37:17-25. - PubMed
-
- Arrecubieta, C., R. Lopez, and E. Garcia. 1994. Molecular Characterization of cap3A, a gene from the operon required for the synthesis of the capsule of Streptococcus pneumoniae type 3: sequencing of mutations responsible for the unencapsulated phenotype and localization of the capsular cluster on the pneumococcal chromosome. J. Bacteriol. 176:6375-6383. - PMC - PubMed
-
- Austrian, R., and J. Gold. 1964. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann. Intern. Med. 60:759-776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

