Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis
- PMID: 16547028
- PMCID: PMC1428413
- DOI: 10.1128/JB.188.7.2421-2426.2006
Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis
Abstract
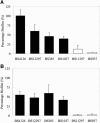
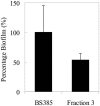
Biofilm formation is an increasing problem in medicine, due to the intrinsic resistance of microorganisms in the biofilm mode of growth against the host immune system and antimicrobial therapy. Adhesion is an important step in biofilm formation, influenced, among other factors, by the surface hydrophobicities and charges of both the substratum and the adhering microorganisms. Enterococcus faecalis strains generally display subpopulations with different surface charges, expressed as bimodal zeta potential distributions. Two-thirds of E. faecalis strains isolated from clogged biliary stents displayed such heterogeneity of surface charges in culture. In this study, the influence of this culture heterogeneity on initial adhesion and subsequent biofilm formation was investigated. Heterogeneous strains were retained in higher numbers on polystyrene than homogeneous strains. Also, biofilm formation was much more pronounced for heterogeneous strains than for homogeneous strains. In a population enriched to display only one subpopulation, fewer bacteria were retained than in its original heterogeneous culture. Also, the enriched subpopulation formed less biofilm than its original heterogeneous culture. The presence of ox bile during adhesion resulted in fewer retained bacteria, although heterogeneous strains were still retained in significantly higher numbers than were homogeneous strains, and, in general, the presence of ox bile reduced biofilm formation. The initial adhesion and biofilm formation were independent of the presence of the gene encoding the enterococcal surface protein (esp) or the expression of gelatinase (GelE). It is concluded that heterogeneity in cell surface charge represents an advantage for bacteria in the colonization of surfaces.
Figures


References
-
- An, Y. H., and R. J. Friedman. 1998. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 43:338-348. - PubMed
-
- Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. - PubMed
-
- Cowan, M. M., H. C. van der Mei, I. Stokroos, and H. J. Busscher. 1992. Heterogeneity of surfaces of subgingival bacteria as detected by zeta potential measurements. J. Dent. Res. 71:1803-1806. - PubMed
-
- Davies, D. G. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114-122. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

