Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases
- PMID: 16547101
- PMCID: PMC1461427
- DOI: 10.1534/genetics.106.055749
Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases
Abstract
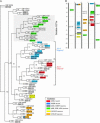
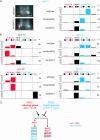
Functional left/right asymmetry ("laterality") is a fundamental feature of many nervous systems, but only very few molecular correlates to functional laterality are known. At least two classes of chemosensory neurons in the nematode Caenorhabditis elegans are functionally lateralized. The gustatory neurons ASE left (ASEL) and ASE right (ASER) are two bilaterally symmetric neurons that sense distinct chemosensory cues and express a distinct set of four known chemoreceptors of the guanylyl cyclase (gcy) gene family. To examine the extent of lateralization of gcy gene expression patterns in the ASE neurons, we have undertaken a genomewide analysis of all gcy genes. We report the existence of a total of 27 gcy genes encoding receptor-type guanylyl cyclases and of 7 gcy genes encoding soluble guanylyl cyclases in the complete genome sequence of C. elegans. We describe the expression pattern of all previously uncharacterized receptor-type guanylyl cyclases and find them to be highly biased but not exclusively restricted to the nervous system. We find that >41% (11/27) of all receptor-type guanylyl cyclases are expressed in the ASE gustatory neurons and that one-third of all gcy genes (9/27) are expressed in a lateral, left/right asymmetric manner in the ASE neurons. The expression of all laterally expressed gcy genes is under the control of a gene regulatory network composed of several transcription factors and miRNAs. The complement of gcy genes in the related nematode C. briggsae differs from C. elegans as evidenced by differences in chromosomal localization, number of gcy genes, and expression patterns. Differences in gcy expression patterns in the ASE neurons of C. briggsae arise from a difference in cis-regulatory elements and trans-acting factors that control ASE laterality. In sum, our results indicate the existence of a surprising multitude of putative chemoreceptors in the gustatory ASE neurons and suggest the existence of a substantial degree of laterality in gustatory signaling mechanisms in nematodes.
Figures







References
-
- Bargmann, C. I., 1998. Neurobiology of the Caenorhabditis elegans genome. Science 282: 2028–2033. - PubMed
-
- Bargmann, C. I., and H. R. Horvitz, 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742. - PubMed
-
- Bargmann, C. I., and I. Mori, 1997. Chemotaxis and thermotaxis, pp. 717–738 in C.elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

