Ionic contrast terahertz near-field imaging of axonal water fluxes
- PMID: 16547134
- PMCID: PMC1458751
- DOI: 10.1073/pnas.0510945103
Ionic contrast terahertz near-field imaging of axonal water fluxes
Erratum in
- Proc Natl Acad Sci U S A. 2007 May 22;104(21):9099
Abstract
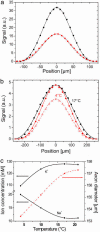
We demonstrate the direct and noninvasive imaging of functional neurons by ionic contrast terahertz near-field microscopy. This technique provides quantitative measurements of ionic concentrations in both the intracellular and extracellular compartments and opens the way to direct noninvasive imaging of neurons during electrical, toxin, or thermal stresses. Furthermore, neuronal activity results from both a precise control of transient variations in ionic conductances and a much less studied water exchange between the extracellular matrix and the intraaxonal compartment. The developed ionic contrast terahertz microscopy technique associated with a full three-dimensional simulation of the axon-aperture near-field system allows a precise measurement of the axon geometry and therefore the direct visualization of neuron swelling induced by temperature change or neurotoxin poisoning. Water influx as small as 20 fl per mum of axonal length can be measured. This technique should then provide grounds for the development of advanced functional neuroimaging methods based on diffusion anisotropy of water molecules.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Mittleman D. in Sensing with Terahertz Radiation. Berlin: Springer; 2003.
-
- Mittleman D. M., Jacobsen R. H., Nuss M. C. IEEE J. Quantum Electron. 1996;2:679–692.
-
- Löffler T., Bauer T., Siebert K. J., Roskos H. G., Fitzgerald A., Czasch S. Opt. Exp. 2001;9:616–621. - PubMed
-
- Fitzgerald A. J., Berry E., Zinovev N. N., Walker G. C., Smith M. A., Chamberlain M. A. Phys. Med. Biol. 2002;47:R67–R84. - PubMed
-
- Woodward R. M., Cole B. E., Wallace V. P., Pye R. J., Arnone D. D., Linfield E. H., Pepper M. Phys. Med. Biol. 2002;47:3853–3863. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

