Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity
- PMID: 16547141
- PMCID: PMC1458814
- DOI: 10.1073/pnas.0507175103
Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity
Abstract
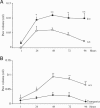
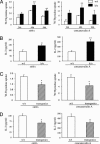
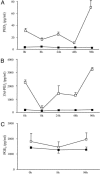
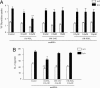
Hematopoietic prostaglandin D(2) synthase (hPGD(2)S) metabolizes cyclooxygenase-derived prostaglandin (PG) H(2) to PGD(2), which is dehydrated to cyclopentenone PGs, including 15-deoxy-Delta(12,14)-PGJ(2) (15d-PGJ(2)). PGD(2) acts through two receptors (DP1 and DP2/CRTH2), whereas 15d-PGJ(2) can activate peroxisome proliferator-activated receptors or inhibit a range of proinflammatory signaling pathways, including NF-kappaB. Despite eliciting asthmatic and allergic reactions through the generation of PGD(2), it is not known what role hPGD(2)S plays in T helper (Th)1-driven adaptive immunity. To investigate this question, the severity and duration of a delayed type hypersensitivity reaction was examined in hPGD(2)S knockout and transgenic mice. Compared with their respective controls, knockouts displayed a more severe inflammatory response that failed to resolve, characterized histologically as persistent acute inflammation, whereas transgenic mice had little detectable inflammation. Lymphocytes isolated from inguinal lymph nodes of hPGD(2)S(-/-) animals showed hyperproliferation and increased IL-2 synthesis effects that were rescued by 15d-PGJ(2), but not PGD(2), working through either of its receptors. Crucially, 15d-PGJ(2) exerted its suppressive effects through the inhibition of NF-kappaB activation and not through peroxisome proliferator-activated receptor signaling. In contrast, lymph node cultures from transgenics proliferated more slowly and synthesized significantly less IL-2 than controls. Therefore, contrary to its role in driving Th2-like responses, this report shows that hPGD(2)S may act as an internal braking signal essential for bringing about the resolution of Th1-driven delayed type hypersensitivity reactions. Consequently, hPGD(2)S-derived cyclopentenone PGs may protect against inflammatory diseases, where T lymphocytes play a pathogenic role, as in rheumatoid arthritis, atopic eczema, and chronic rejection.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
-
- Flower R. J. Pharmacol. Rev. 1974;26:33–67. - PubMed
-
- Gilroy D. W., Lawrence T., Perretti M., Rossi A. G. Nat. Rev. Drug Discov. 2004;3:401–416. - PubMed
-
- Serhan C. N. Histochem. Cell Biol. 2004;122:305–321. - PubMed
-
- Gilroy D. W., Colville-Nash P. R., Willis D., Chivers J., Paul-Clark M. J., Willoughby D. A. Nat. Med. 1999;5:698–701. - PubMed
-
- Luster A. D., Tager A. M. Nat. Rev. Immunol. 2004;4:711–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

