Specific sequence motifs direct the oxygenation and chlorination of tryptophan by myeloperoxidase
- PMID: 16548523
- PMCID: PMC2556706
- DOI: 10.1021/bi052339+
Specific sequence motifs direct the oxygenation and chlorination of tryptophan by myeloperoxidase
Abstract
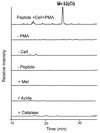
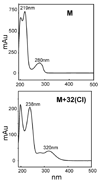
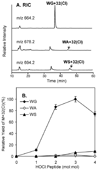
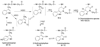
Most studies of protein oxidation have typically focused on the reactivity of single amino acid side chains while ignoring the potential importance of adjacent sequences in directing the reaction pathway. We previously showed that hypochlorous acid (HOCl), a specific product of myeloperoxidase, inactivates matrilysin by modifying adjacent tryptophan and glycine (WG) residues in the catalytic domain. Here, we use model peptides that mimic the region of matrilysin involved in this reaction, VVWGTA, VVWATA, and the library VVWXTA, to determine whether specific sequence motifs are targeted for chlorination or oxygenation by myeloperoxidase. Our results demonstrate that HOCl generated by myeloperoxidase or activated neutrophils converts the peptide VVWGTA to a chlorinated product, WG+32(Cl). Tandem mass spectrometry in concert with high resolution 1H and two-dimensional NMR analysis revealed that the modification required cross-linking of the tryptophan to the amide of glycine followed by chlorination of the indole ring of tryptophan. In contrast, when glycine in the peptide was replaced with alanine, the major products were mono- and dioxygenated tryptophan residues. When the peptide library VVWXTA (where X represents all 20 common amino acids) was exposed to HOCl, only WG produced a high yield of the chloroindolenine derivative. However, when glycine was replaced by other amino acids, oxygenated tryptophan derivatives were the major products. Our observations indicate that WG may represent a specific sequence motif in proteins that is targeted for chlorination by myeloperoxidase.
Figures





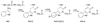
References
-
- Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. - PubMed
-
- Klebanoff SJ, Clark RA. The neutrophil: functional and clinical disorders. New York: North-Holland Pub. Co.; 1978.
-
- Hurst JK, Barrette WC., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24:271–328. - PubMed
-
- Dinauer MC. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci. 1993;30:329–369. - PubMed
-
- Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol. 2000;7:53–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

