A "traffic control" role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing
- PMID: 16549496
- PMCID: PMC2063766
- DOI: 10.1083/jcb.200507111
A "traffic control" role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing
Abstract
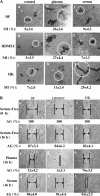
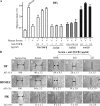
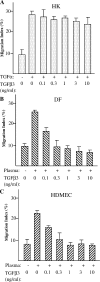
Cell migration is a rate-limiting event in skin wound healing. In unwounded skin, cells are nourished by plasma. When skin is wounded, resident cells encounter serum for the first time. As the wound heals, the cells experience a transition of serum back to plasma. In this study, we report that human serum selectively promotes epidermal cell migration and halts dermal cell migration. In contrast, human plasma promotes dermal but not epidermal cell migration. The on-and-off switch is operated by transforming growth factor (TGF) beta3 levels, which are undetectable in plasma and high in serum, and by TGFbeta receptor (TbetaR) type II levels, which are low in epidermal cells and high in dermal cells. Depletion of TGFbeta3 from serum converts serum to a plasmalike reagent. The addition of TGFbeta3 to plasma converts it to a serumlike reagent. Down-regulation of TbetaRII in dermal cells or up-regulation of TbetaRII in epidermal cells reverses their migratory responses to serum and plasma, respectively. Therefore, the naturally occurring plasma-->serum-->plasma transition during wound healing orchestrates the orderly migration of dermal and epidermal cells.
Figures





References
-
- Albrecht-Buehler, G. 1977. The phagokinetic tracks of 3T3 cells. Cell. 11:395–404. - PubMed
-
- Ashcroft, G.S., X. Yang, A.B. Glick, M. Weinsteil, J.L. Letterio, D.E. Mizel, M. Anzano, T. Greenwell-Wild, S.M. Wahl, C. Deng, and A.B. Roberts. 1999. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1:260–266. - PubMed
-
- Assoian, R.K., A. Komoriya, C.A. Meyers, D.M. Miller, and M.B. Sporn. 1983. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 258:7155–7160. - PubMed
-
- Assoian, R.K., G.R. Grotendorst, D.M. Miller, and M.B. Sporn. 1984. Cellular transformation by coordinated action of three peptide growth factors from human platelets. Nature. 309:804–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

