GPI-anchored proteins are directly targeted to the apical surface in fully polarized MDCK cells
- PMID: 16549497
- PMCID: PMC2063760
- DOI: 10.1083/jcb.200507116
GPI-anchored proteins are directly targeted to the apical surface in fully polarized MDCK cells
Abstract
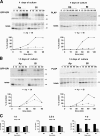
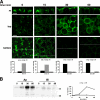
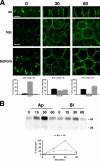
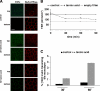
The polarity of epithelial cells is dependent on their ability to target proteins and lipids in a directional fashion. The trans-Golgi network, the endosomal compartment, and the plasma membrane act as sorting stations for proteins and lipids. The site of intracellular sorting and pathways used for the apical delivery of glycosylphosphatidylinositol (GPI)-anchored proteins (GPI-APs) are largely unclear. Using biochemical assays and confocal and video microscopy in living cells, we show that newly synthesized GPI-APs are directly delivered to the apical surface of fully polarized Madin-Darby canine kidney cells. Impairment of basolateral membrane fusion by treatment with tannic acid does not affect the direct apical delivery of GPI-APs, but it does affect the organization of tight junctions and the integrity of the monolayer. Our data clearly demonstrate that GPI-APs are directly sorted to the apical surface without passing through the basolateral membrane. They also reinforce the hypothesis that apical sorting of GPI-APs occurs intracellularly before arrival at the plasma membrane.
Figures


Comment in
-
Controversy fuels trafficking of GPI-anchored proteins.J Cell Biol. 2006 Mar 27;172(7):963-5. doi: 10.1083/jcb.200603015. J Cell Biol. 2006. PMID: 16567497 Free PMC article.
References
-
- Apodaca, G., and K.E. Mostov. 1993. Transcytosis of placental alkaline phosphatase-polymeric immunoglobulin receptor fusion proteins is regulated by mutations of Ser664. J. Biol. Chem. 268:23712–23719. - PubMed
-
- Arreaza, G., and D.A. Brown. 1995. Sorting and intracellular trafficking of a glycosylphosphatidylinositol-anchored protein and two hybrid transmembrane proteins with the same ectodomain in Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 270:23641–23647. - PubMed
-
- Bonifacino, J.S., and L.M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

