Experimental determination of the radiation dose limit for cryocooled protein crystals
- PMID: 16549763
- PMCID: PMC1458769
- DOI: 10.1073/pnas.0600973103
Experimental determination of the radiation dose limit for cryocooled protein crystals
Abstract
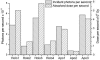
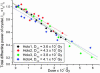
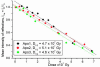
Radiation damage to cryocooled protein crystals during x-ray structure determination has become an inherent part of macromolecular diffraction data collection at third-generation synchrotrons. Generally, radiation damage is an undesirable component of the experiment and can result in erroneous structural detail in the final model. The characterization of radiation damage thus has become an important area for structural biologists. The calculated dose limit of 2 x 10(7) Gy for the diffracting power of cryocooled protein crystals to drop by half has been experimentally evaluated at a third-generation synchrotron source. Successive data sets were collected from four holoferritin and three apoferritin crystals. The absorbed dose for each crystal was calculated by using the program raddose after measurement of the incident photon flux and determination of the elemental crystal composition by micro-particle-induced x-ray emission. Degradation in diffraction quality and specific structural changes induced by synchrotron radiation then could be compared directly with absorbed dose for different dose/dose rate regimes: a 10% lifetime decrease for a 10-fold dose rate increase was observed. Remarkable agreement both between different crystals of the same type and between apoferritin and holoferritin was observed for the dose required to reduce the diffracted intensity by half (D(1/2)). From these measurements, a dose limit of D(1/2) = 4.3 (+/-0.3) x10(7) Gy was obtained. However, by considering other data quality indicators, an intensity reduction to I(ln2) = ln2 x I(0), corresponding to an absorbed dose of 3.0 x 10(7) Gy, is recommended as an appropriate dose limit for typical macromolecular crystallography experiments.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Blake C., Phillips D. C. Proceedings of the Symposium on the Biological Effects of Ionizing Radiation at the Molecular Level; Vienna: International Atomic Energy Agency; 1962. pp. 183–191.
-
- Haas D., Rossmann M. G. Acta Crystallogr. B. 1970;26:998–1004. - PubMed
-
- Garman E. F., Schneider T. R. J. Appl. Cryst. 1997;27:211–237.
-
- Rodgers D. W. Methods Enzymol. 1997;276:183–202. - PubMed
-
- Burmeister W. P. Acta Crystallogr. D. 2000;56:328–341. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

