Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease
- PMID: 16549764
- PMCID: PMC1405622
- DOI: 10.1073/pnas.0600948103
Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease
Abstract
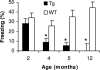
Alzheimer's disease (AD) is a progressive neurodegenerative disorder for which numerous mouse models have been generated. In both AD patients and mouse models, there is increasing evidence that neuronal dysfunction occurs before the accumulation of beta-amyloid (Abeta)-containing plaques and neurodegeneration. Characterization of the timing and nature of preplaque dysfunction is important for understanding the progression of this disease and to identify pathways and molecular targets for therapeutic intervention. Hence, we have examined the progression of dysfunction at the morphological, functional, and behavioral levels in the Tg2576 mouse model of AD. Our data show that decreased dendritic spine density, impaired long-term potentiation (LTP), and behavioral deficits occurred months before plaque deposition, which was first detectable at 18 months of age. We detected a decrease in spine density in the outer molecular layer of the dentate gyrus (DG) beginning as early as 4 months of age. Furthermore, by 5 months, there was a decline in LTP in the DG after perforant path stimulation and impairment in contextual fear conditioning. Moreover, an increase in the Abeta42/Abeta40 ratio was first observed at these early ages. However, total amyloid levels did not significantly increase until approximately 18 months of age, at which time significant increases in reactive astrocytes and microglia could be observed. Overall, these data show that the perforant path input from the entorhinal cortex to the DG is compromised both structurally and functionally, and this pathology is manifested in memory defects long before significant plaque deposition.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



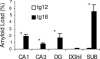

References
-
- Selkoe D. J. Ann. N.Y. Acad. Sci. 2000;924:17–25. - PubMed
-
- Hardy J., Selkoe D. J. Science. 2002;297:353–356. - PubMed
-
- Bloom G. S., Ren K., Glabe C. G. Biochim. Biophys. Acta. 2005;1739:116–124. - PubMed
-
- Ashe K. H. Learn. Mem. 2001;8:301–308. - PubMed
-
- Morgan D. Neurochem. Res. 2003;28:1029–1034. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

