Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene
- PMID: 16549777
- PMCID: PMC1458798
- DOI: 10.1073/pnas.0600548103
Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene
Erratum in
- Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10526
-
Correction for Wani et al., Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant β2-microglobulin gene.Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10526. doi: 10.1073/pnas.0604332103. Epub 2006 Jun 20. Proc Natl Acad Sci U S A. 2006. PMID: 29507150 Free PMC article. No abstract available.
Abstract
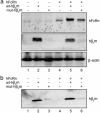
Two siblings, products of a consanguineous marriage, were markedly deficient in both albumin and IgG because of rapid degradation of these proteins, suggesting a lack of the neonatal Fc receptor, FcRn. FcRn is a heterodimeric receptor composed of a nonclassical MHC class I alpha-chain and beta(2)-microglobulin (beta(2)m) that binds two ligands, IgG and albumin, and extends the catabolic half-lives of both. Eight relatives of the siblings were moderately IgG-deficient. From sera archived for 35 years, we sequenced the two siblings' genes for the heterodimeric FcRn. We found that, although the alpha-chain gene sequences of the siblings were normal, the beta(2)m genes contained a single nucleotide transversion that would mutate a conserved alanine to proline at the midpoint of the signal sequence. Concentrations of soluble beta(2)m and HLA in the siblings' sera were <1% of normal. Transfection assays of beta(2)m-deficient cultured cells with beta(2)m cDNA indicated that the mutant beta(2)m supported <20% of normal expression of beta(2)m, MHC class I, and FcRn proteins. We concluded that a beta(2)m gene mutation underlies the hypercatabolism and reduced serum levels of albumin and IgG in the two siblings with familial hypercatabolic hypoproteinemia. This experiment of nature affirms our hypothesis that FcRn binds IgG and albumin, salvages both from a degradative fate, and maintains their physiologic concentrations.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

