Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia
- PMID: 16549778
- PMCID: PMC1405626
- DOI: 10.1073/pnas.0600506103
Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia
Abstract
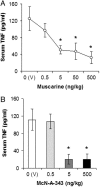
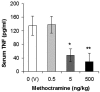
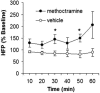
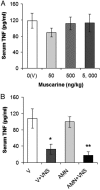
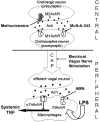
TNF has a critical mediator role in inflammation and is an important therapeutic target. We recently discovered that TNF production is regulated by neural signals through the vagus nerve. Activation of this "cholinergic antiinflammatory pathway" inhibits the production of TNF and other cytokines and protects animals from the inflammatory damage caused by endotoxemia and severe sepsis. Here, we describe a role for central muscarinic acetylcholine receptors in the activation of the cholinergic antiinflammatory pathway. Central muscarinic cholinergic activation by muscarine, the M1 receptor agonist McN-A-343, and the M2 receptor antagonist methoctramine inhibited serum TNF levels significantly during endotoxemia. Centrally administered methoctramine stimulated vagus-nerve activity measured by changes in instantaneous heart-rate variability. Blockade of peripheral muscarinic receptors did not abolish antiinflammatory signaling through the vagus nerve, indicating that peripheral muscarinic receptors on immune cells are not required for the cytokine-regulating activities of the cholinergic antiinflammatory pathway. The role of central muscarinic receptors in activating the cholinergic antiinflammatory pathway is of interest for the use of centrally acting muscarinic cholinergic enhancers as antiinflammatory agents.
Conflict of interest statement
Conflict of interest statement: K.J.T. and V.A.P. are inventors on patents related to the content of this paper. K.J.T. is a founder and consultant to Critical Therapeutics, Inc.
Figures
References
-
- Tracey K. J. Nature. 2002;420:853–859. - PubMed
-
- Tracey K. J. Fatal Sequence: The Killer Within. Washington, DC: Dana; 2005. pp. 128–204.
-
- Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R., Wang H., Abumrad N., Eaton J. W., Tracey K. J. Nature. 2000;405:458–461. - PubMed
-
- Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., et al. Nature. 2003;421:384–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

