Galectin-3 regulates myofibroblast activation and hepatic fibrosis
- PMID: 16549783
- PMCID: PMC1458794
- DOI: 10.1073/pnas.0511167103
Galectin-3 regulates myofibroblast activation and hepatic fibrosis
Abstract
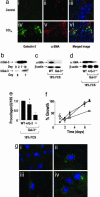
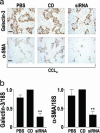
Central to fibrogenesis and the scarring of organs is the activation of fibroblasts into matrix-secreting myofibroblasts. We demonstrate that Galectin-3 expression is up-regulated in established human fibrotic liver disease and is temporally and spatially related to the induction and resolution of experimental hepatic fibrosis. Disruption of the Galectin-3 gene blocks myofibroblast activation and procollagen (I) expression in vitro and in vivo, markedly attenuating liver fibrosis. Addition of exogenous recombinant Galectin-3 in vitro reversed this abnormality. The reduction in hepatic fibrosis observed in the Galectin-3(-/-) mouse occurred despite equivalent liver injury and inflammation, and similar tissue expression of TGF-beta. TGF-beta failed to transactivate Galectin-3(-/-) hepatic stellate cells, in contrast with WT hepatic stellate cells; however, TGF-beta-stimulated Smad-2 and -3 activation was equivalent. These data suggest that Galectin-3 is required for TGF-beta mediated myofibroblast activation and matrix production. Finally, in vivo siRNA knockdown of Galectin-3 inhibited myofibroblast activation after hepatic injury and may therefore provide an alternative therapeutic approach to the prevention and treatment of liver fibrosis.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Neuberger J. J. Hepatol. 2000;32:198–207. - PubMed
-
- Simpson K. J., Garden O. J. Proc. R. Coll. Physicians Edinb.; 1999. pp. 144–152.
-
- Friedman S. L. J. Biol. Chem. 2000;275:2247–2250. - PubMed
-
- Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K., et al. Cell. 1994;76:597–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

