N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk
- PMID: 16550165
- PMCID: PMC1434721
- DOI: 10.1038/sj.onc.1209374
N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk
Abstract
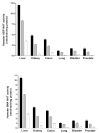
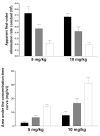
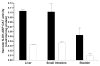
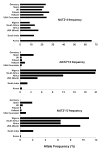
A role for the N-acetyltransferase 2 (NAT2) genetic polymorphism in cancer risk has been the subject of numerous studies. Although comprehensive reviews of the NAT2 acetylation polymorphism have been published elsewhere, the objective of this paper is to briefly highlight some important features of the NAT2 acetylation polymorphism that are not universally accepted to better understand the role of NAT2 polymorphism in carcinogenic risk assessment. NAT2 slow acetylator phenotype(s) infer a consistent and robust increase in urinary bladder cancer risk following exposures to aromatic amine carcinogens. However, identification of specific carcinogens is important as the effect of NAT2 polymorphism on urinary bladder cancer differs dramatically between monoarylamines and diarylamines. Misclassifications of carcinogen exposure and NAT2 genotype/phenotype confound evidence for a real biological effect. Functional understanding of the effects of NAT2 genetic polymorphisms on metabolism and genotoxicity, tissue-specific expression and the elucidation of the molecular mechanisms responsible are critical for the interpretation of previous and future human molecular epidemiology investigations into the role of NAT2 polymorphism on cancer risk. Although associations have been reported for various cancers, this paper focuses on urinary bladder cancer, a cancer in which a role for NAT2 polymorphism was first proposed and for which evidence is accumulating that the effect is biologically significant with important public health implications.
Figures




References
-
- Abe M, Deguchi T, Suzuki T. Biochem Biophys Res Commun. 1993;191:811–6. - PubMed
-
- Agundez JA, Menaya JG, Tejeda R, Lago F, Chavez M, Benitez J. Pharmacogenetics. 1996;6:465–72. - PubMed
-
- Airoldi L, Orsi F, Magagnotti C, Coda R, Randone D, Casetta G, Peluso M, Hautefeuille A, Malaveille C, Vineis P. Carcinogenesis. 2002;23:861–6. - PubMed
-
- Anitha A, Banerjee M. Int J Mol Med. 2003;11:125–31. - PubMed
-
- Badawi AF, Hirvonen A, Bell DA, Lang NP, Kadlubar FF. Cancer Res. 1995;55:5230–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

