The alkaloid alstonine: a review of its pharmacological properties
- PMID: 16550222
- PMCID: PMC1375234
- DOI: 10.1093/ecam/nek011
The alkaloid alstonine: a review of its pharmacological properties
Abstract
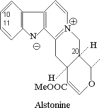
Indole compounds, related to the metabolism of tryptophan, constitute an extensive family, and are found in bacteria, plants and animals. Indolic compounds possess significant and complex physiological roles, and especially indole alkaloids have historically constituted a class of major importance in the development of new plant derived drugs. The indole alkaloid alstonine has been identified as the major component of a plant-based remedy, used in Nigeria to treat mental illnesses by traditional psychiatrists. Although it is certainly difficult to compare the very concept of mental disorders in different cultures, the traditional use of alstonine is remarkably compatible with its profile in experimental animals. Even though alstonine in mice models shows a psychopharmacological profile closer to the newer atypical antipsychotic agents, it also shows important differences and what seems to be an exclusive mechanism of action, not entirely clarified at this point. Considering the seemingly unique mode of action of alstonine and that its traditional use can be viewed as indicative of bioavailability and safety, this review focuses on the effects of alstonine in the central nervous system, particularly on its unique profile as an antipsychotic agent. We suggest that a thorough understanding of traditional medical concepts of health and disease in general and traditional medical practices in particular, can lead to true innovation in paradigms of drug action and development. Overall, the study of this unique indole alkaloid may be considered as another example of the richness of medicinal plants and traditional medical systems in the discovery of new prototypic drugs.
Figures









References
-
- Williams JE. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on uña de gato and sangre de grado. Altern. Med. Rev. 2001;6:567–79. - PubMed
-
- Cano A, Alcaraz O, Arnao MB. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal Bioanal Chem. 2003;376:33–7. - PubMed
-
- Cordell GA, Quinn-Beattie ML, Farnsworth NR. The potencial of alkaloids in drug discovery. Phytother Res. 2001;15:183–205. - PubMed
-
- Costa-Campos L, Elisabetsky E, Lara DR, Carlson TJ, King SR, Ubilas R, et al. Antipsychotic profile of alstonine: ethnopharmacology of a traditional Nigerian botanical remedy. An Acad Bras Cienc. 1999;71:189–201. - PubMed
-
- Iwu MM, Klayman DL. Evaluation of the in vitro antimalarial activity of Picralima nitida extracts. J Ethnopharmacol. 1992;36:133–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

